Czech Republic Orthopedic Devices Market Outlook | Growth, Revenue, Share, Analysis, Value, Industry, Companies, Trends, Size, COVID-19 IMPACT & Forecast
| Product Code: ETC368116 | Publication Date: Aug 2022 | Updated Date: Jul 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Czech Republic Orthopedic Devices Market Size Growth Rate
The Czech Republic Orthopedic Devices Market is poised for steady growth rate improvements from 2025 to 2029. The growth rate starts at -0.09% in 2025 and reaches 7.59% by 2029.

Orthopedic Devices Market: Czech Republic vs Top 5 Major Economies in 2027 (Europe)
By 2027, the Orthopedic Devices market in Czech Republic is anticipated to reach a growth rate of 2.54%, as part of an increasingly competitive Europe region, where Germany remains at the forefront, supported by United Kingdom, France, Italy and Russia, driving innovations and market adoption across sectors.
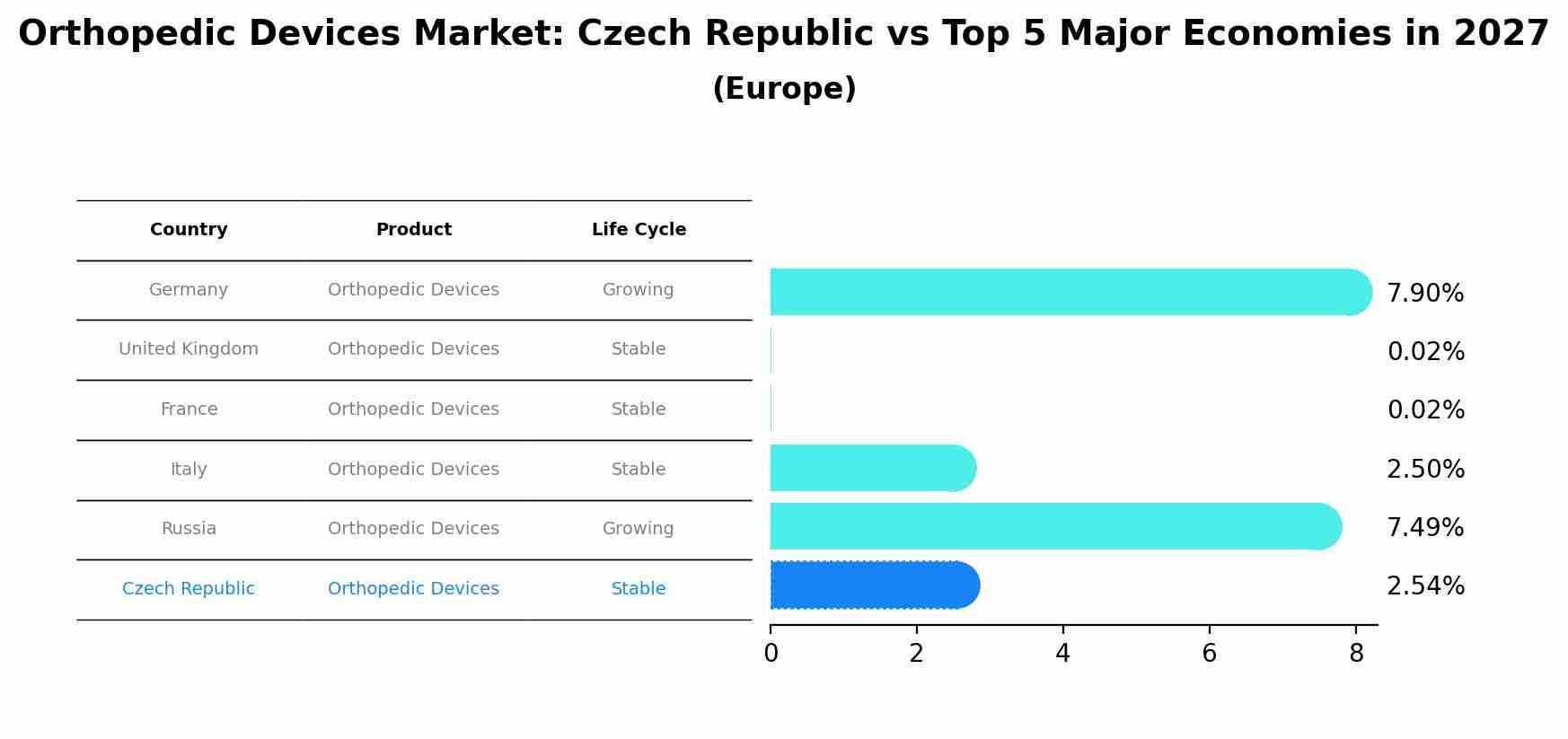
Czech Republic Orthopedic Devices Market Synopsis
The Czech Republic Orthopedic Devices Market is characterized by a growing demand for advanced orthopedic implants and devices due to an aging population and increasing incidences of orthopedic disorders. Key market players include multinational companies and local manufacturers offering a wide range of products such as joint implants, spinal devices, trauma fixation devices, and orthobiologics. The market is driven by technological advancements, rising healthcare expenditure, and a focus on improving patient outcomes. Regulatory reforms and reimbursement policies also play a significant role in shaping the market landscape. Market growth is further supported by collaborations between healthcare providers and industry stakeholders to enhance the quality of orthopedic care in the country. Overall, the Czech Republic Orthopedic Devices Market presents opportunities for innovation and market expansion.
Czech Republic Orthopedic Devices Market Trends
In the Czech Republic Orthopedic Devices Market, there is a growing demand for minimally invasive procedures and advanced technology such as robotic-assisted surgeries. This trend is driven by the increasing prevalence of orthopedic conditions among the aging population and the desire for quicker recovery times and reduced post-operative complications. Additionally, there is a rising focus on personalized orthopedic solutions tailored to individual patient needs, leading to the development of custom implants and 3D printing technologies. The market is also witnessing a shift towards value-based healthcare models, with a greater emphasis on cost-effectiveness and improved patient outcomes. Overall, the Czech Republic Orthopedic Devices Market is evolving towards more innovative and patient-centric approaches to orthopedic care.
Czech Republic Orthopedic Devices Market Challenges
The Czech Republic Orthopedic Devices Market faces several challenges, including increasing competition from international manufacturers, pricing pressures due to government budget constraints, and limited reimbursement coverage for certain advanced orthopedic treatments. Additionally, regulatory hurdles and slow adoption of new technologies in some healthcare facilities hinder market growth. Furthermore, the relatively small market size compared to other European countries poses challenges for companies aiming to achieve economies of scale. To succeed in this market, companies need to navigate these challenges by offering innovative solutions, developing cost-effective products, and establishing strong partnerships with local healthcare providers to ensure market access and uptake of orthopedic devices.
Czech Republic Orthopedic Devices Market Investment Opportunities
The Czech Republic orthopedic devices market presents promising investment opportunities driven by factors such as a growing elderly population, increasing prevalence of orthopedic disorders, and advancements in medical technology. Key segments within the market include joint reconstruction, orthobiologics, spinal devices, and trauma fixation. With a strong healthcare infrastructure and rising healthcare expenditure, there is a demand for innovative orthopedic devices that offer better outcomes and improved patient care. Investing in companies that specialize in developing cutting-edge orthopedic implants, instruments, and technologies could prove to be lucrative in the Czech Republic market. Additionally, partnerships with local healthcare providers, hospitals, and distributors can help in establishing a strong presence and capturing market share in this evolving sector.
Jordan Agar Market Government Policies
In the Czech Republic, orthopedic devices are regulated by the State Institute for Drug Control (SUKL) under the Medical Devices Act. The government requires orthopedic devices to meet European Union regulations for safety and quality standards, ensuring that only approved products are marketed in the country. Manufacturers must obtain CE marking to demonstrate compliance with these standards. Additionally, reimbursement for orthopedic devices is provided through the public health insurance system, with pricing controlled by the Czech Ministry of Health. This regulatory framework aims to protect patient safety, promote innovation in the orthopedic devices market, and ensure accessibility to necessary medical devices for the population of the Czech Republic.
Czech Republic Orthopedic Devices Market Future Outlook
The Czech Republic Orthopedic Devices Market is expected to exhibit steady growth in the coming years due to the increasing prevalence of orthopedic conditions, such as osteoarthritis and fractures, in the aging population. Technological advancements in orthopedic devices, such as implants and prosthetics, will drive market growth by providing more effective treatment options. Additionally, the rising awareness about the benefits of early intervention and the availability of advanced healthcare facilities in the Czech Republic will further boost the demand for orthopedic devices. However, pricing pressures and reimbursement challenges may pose some limitations to market growth. Overall, the Czech Republic Orthopedic Devices Market is anticipated to expand at a moderate pace, driven by demographic trends and technological innovations.
Key Highlights of the Report:
- Czech Republic Orthopedic Devices Market Outlook
- Market Size of Czech Republic Orthopedic Devices Market, 2021
- Forecast of Czech Republic Orthopedic Devices Market, 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Revenues & Volume for the Period 2018 - 2031
- Czech Republic Orthopedic Devices Market Trend Evolution
- Czech Republic Orthopedic Devices Market Drivers and Challenges
- Czech Republic Orthopedic Devices Price Trends
- Czech Republic Orthopedic Devices Porter's Five Forces
- Czech Republic Orthopedic Devices Industry Life Cycle
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Application for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Hip Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Knee Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Spine Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Craniomaxillofacial Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Dental Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Sports Injuries, Extremities And Trauma (Set) Orthopedic Devices for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Product for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Drill Guide for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Guide Tubes for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Implant Holder for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Custom Clamps for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Distracters for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Screw Drivers for the Period 2018 - 2031
- Historical Data and Forecast of Czech Republic Orthopedic Devices Market Revenues & Volume By Accessories for the Period 2018 - 2031
- Czech Republic Orthopedic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By Product
- Czech Republic Orthopedic Devices Top Companies Market Share
- Czech Republic Orthopedic Devices Competitive Benchmarking By Technical and Operational Parameters
- Czech Republic Orthopedic Devices Company Profiles
- Czech Republic Orthopedic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













