India Medical Device Testing Market (2025-2031) Outlook | Size, Share, Analysis, Industry, Revenue, Forecast, Value, Companies, Trends & Growth
| Product Code: ETC4589905 | Publication Date: Jul 2023 | Product Type: Report | ||
| Publisher: 6Wresearch | Author: Ravi Bhandari | No. of Pages: 85 | No. of Figures: 45 | No. of Tables: 25 |
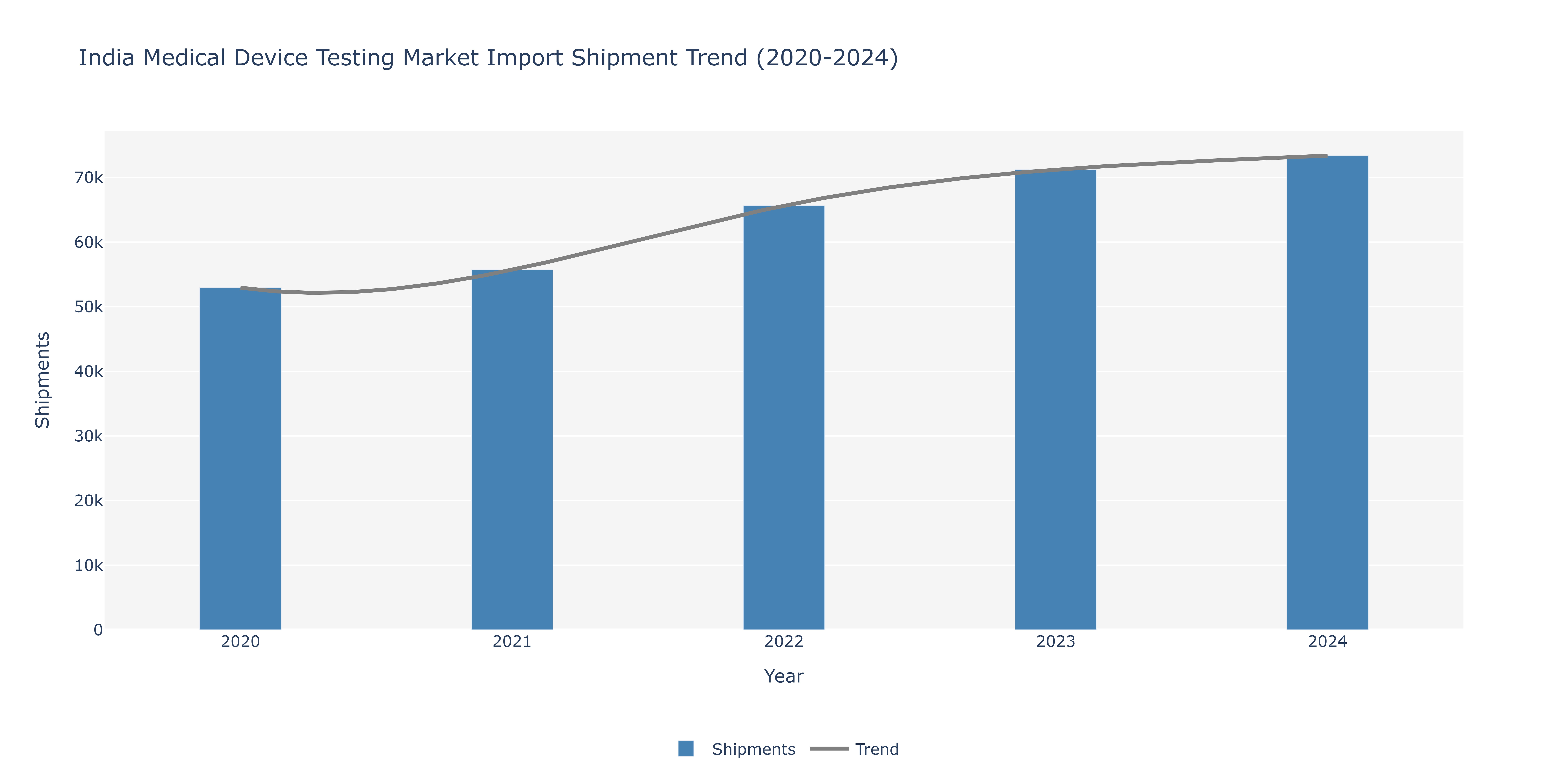
India Medical Device Testing Market Import Shipment Trend (2020-2024)
The India medical device testing market import shipments demonstrated a consistent growth trend with a CAGR of 8.5% from 2020 to 2024. However, there was a slight slowdown in growth with a 3.0% rate between 2023 and 2024. Overall, the market expanded steadily, indicating a positive momentum despite the marginal dip in growth rate towards the end of the period.
India Medical Device Testing Market Overview
India medical device testing market is expanding due to the increasing demand for rigorous testing and certification of medical equipment. With the growth of the healthcare sector and the development of new medical technologies, there is a rising need for ensuring the safety, efficacy, and compliance of medical devices through testing and certification processes.
Drivers of the Market
The India Medical Device Testing market is witnessing robust growth due to several key drivers. Firstly, the global medical device industry has been expanding rapidly, and India is becoming a significant player in the manufacturing and export of medical devices. To ensure these products meet international standards and safety requirements, rigorous testing and certification are essential, which boosts the demand for testing services. Additionally, India growing healthcare sector and the increasing awareness among patients about the quality and safety of medical devices are driving factors. Regulatory compliance and the need to meet the stringent requirements of international markets further underscore the importance of testing. The COVID-19 pandemic has also highlighted the importance of reliable medical devices, stimulating the demand for testing and certification services in this sector.
Challenges of the Market
The India medical device testing market faces challenges related to regulatory compliance, as evolving standards and the need for rigorous testing to ensure patient safety often result in delays in product approval. Additionally, there`s a lack of standardized testing protocols and infrastructure, leading to inconsistencies and a need for improved testing facilities across the country.
COVID-19 Impact on the Market
The COVID-19 pandemic had a twofold effect on the medical device testing market in India. While testing and certification related to medical devices experienced increased demand, there were logistical challenges and delays due to the overwhelming demand for COVID-19-related medical equipment. This led to a temporary shift in priorities and resource allocation within the industry.
Key Players in the Market
Key players in this domain comprise Eurofins Scientific, SGS SA, T?V S?D, and Toxikon Corporation.
Key Highlights of the Report:
- India Medical Device Testing Market Outlook
- Market Size of India Medical Device Testing Market, 2024
- Forecast of India Medical Device Testing Market, 2031
- Historical Data and Forecast of India Medical Device Testing Revenues & Volume for the Period 2021-2031
- India Medical Device Testing Market Trend Evolution
- India Medical Device Testing Market Drivers and Challenges
- India Medical Device Testing Price Trends
- India Medical Device Testing Porter's Five Forces
- India Medical Device Testing Industry Life Cycle
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Services for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Testing for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Inspection for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Certification for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Technology for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Active Implant for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By IVD for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Orthopedic & Dental for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Opthalmic for the Period 2021-2031
- Historical Data and Forecast of India Medical Device Testing Market Revenues & Volume By Vascular for the Period 2021-2031
- India Medical Device Testing Import Export Trade Statistics
- Market Opportunity Assessment By Services
- Market Opportunity Assessment By Technology
- India Medical Device Testing Top Companies Market Share
- India Medical Device Testing Competitive Benchmarking By Technical and Operational Parameters
- India Medical Device Testing Company Profiles
- India Medical Device Testing Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 India Medical Device Testing Market Overview |
3.1 India Country Macro Economic Indicators |
3.2 India Medical Device Testing Market Revenues & Volume, 2021 & 2031F |
3.3 India Medical Device Testing Market - Industry Life Cycle |
3.4 India Medical Device Testing Market - Porter's Five Forces |
3.5 India Medical Device Testing Market Revenues & Volume Share, By Services, 2021 & 2031F |
3.6 India Medical Device Testing Market Revenues & Volume Share, By Technology, 2021 & 2031F |
4 India Medical Device Testing Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing demand for medical devices in India |
4.2.2 Rising focus on quality and safety standards in the healthcare sector |
4.2.3 Growth in research and development activities in the medical device industry |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for medical device testing |
4.3.2 Lack of standardized testing protocols |
4.3.3 Limited infrastructure and resources for testing facilities |
5 India Medical Device Testing Market Trends |
6 India Medical Device Testing Market, By Types |
6.1 India Medical Device Testing Market, By Services |
6.1.1 Overview and Analysis |
6.1.2 India Medical Device Testing Market Revenues & Volume, By Services, 2021-2031F |
6.1.3 India Medical Device Testing Market Revenues & Volume, By Testing, 2021-2031F |
6.1.4 India Medical Device Testing Market Revenues & Volume, By Inspection , 2021-2031F |
6.1.5 India Medical Device Testing Market Revenues & Volume, By Certification, 2021-2031F |
6.2 India Medical Device Testing Market, By Technology |
6.2.1 Overview and Analysis |
6.2.2 India Medical Device Testing Market Revenues & Volume, By Active Implant, 2021-2031F |
6.2.3 India Medical Device Testing Market Revenues & Volume, By IVD, 2021-2031F |
6.2.4 India Medical Device Testing Market Revenues & Volume, By Orthopedic & Dental, 2021-2031F |
6.2.5 India Medical Device Testing Market Revenues & Volume, By Opthalmic, 2021-2031F |
6.2.6 India Medical Device Testing Market Revenues & Volume, By Vascular, 2021-2031F |
7 India Medical Device Testing Market Import-Export Trade Statistics |
7.1 India Medical Device Testing Market Export to Major Countries |
7.2 India Medical Device Testing Market Imports from Major Countries |
8 India Medical Device Testing Market Key Performance Indicators |
8.1 Number of medical device testing facilities accredited by regulatory bodies |
8.2 Percentage increase in RD investment in the medical device industry |
8.3 Adoption rate of international quality standards for medical device testing |
9 India Medical Device Testing Market - Opportunity Assessment |
9.1 India Medical Device Testing Market Opportunity Assessment, By Services, 2021 & 2031F |
9.2 India Medical Device Testing Market Opportunity Assessment, By Technology, 2021 & 2031F |
10 India Medical Device Testing Market - Competitive Landscape |
10.1 India Medical Device Testing Market Revenue Share, By Companies, 2024 |
10.2 India Medical Device Testing Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















