Sri Lanka In-Vitro Diagnostics Market (2025-2031) | Analysis, Supply, Consumer Insights, Companies, Demand, Pricing Analysis, Forecast, Share, Revenue, Trends, Competition, Restraints, Segments, Value, Challenges, Opportunities, Growth, Segmentation, Strategy, Strategic Insights, Industry, Drivers, Investment Trends, Outlook, Competitive, Size
Market Forecast By Product (Instruments, Reagents & kits, Software & Services), By Type (Clinical Chemistry, Molecular Diagnostics, Immuno Diagnostics, Haematology, Others (Urinalysis, Coagulation etc.)), By Application (Infectious Disease, Diabetes, Cancer/Oncology, Cardiology, Autoimmune Disease, Nephrology, Others (Pulmonology, Gastroenterology, Haematology etc,.)), By End-User (Diagnostic Laboratories, Hospitals and Clinics, Others (Pharmacies, Public health centers etc.)), And Competitive Landscape
| Product Code: ETC11273004 | Publication Date: Apr 2025 | Updated Date: Dec 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 78 | No. of Figures: 26 | No. of Tables: 5 |
Topics Covered in Sri Lanka In-vitro Diagnostics Market Report
Sri Lanka In-vitro Diagnostics Market Report thoroughly covers the market by product, type, application and end user. Sri Lanka In-vitro Diagnostics Market Outlook report provides an unbiased and detailed analysis of the ongoing Sri Lanka In-vitro Diagnostics Market trends, opportunities/high growth areas, and market drivers. This would help stakeholders devise and align their market strategies according to the current and future market dynamics.
Sri Lanka In-vitro Diagnostics Market Synopsis
Sri Lanka In-vitro Diagnostics (IVD) market experienced steady growth in recent years, driven by rising healthcare utilization, and increasing reliance on diagnostic evidence in clinical decision-making. Moroever, the sharp rise in disease burden directly amplified diagnostic demand: malaria cases increased from 2022 to 2023, while tuberculosis cases also rose . Each additional infectious case requires multiple diagnostic interactions, initial screening, confirmation, treatment monitoring, and relapse detection, which significantly boosts consumption of reagents, rapid tests, microscopy supplies, and molecular diagnostics.
As a result, higher case volumes translated into a clear surge in test throughput across public and private labs, expanding overall IVD market value. Additionally, total hospitalizations increased from 2022 to 2023, signalling a heavier patient load across the healthcare system. This automatically pushed up demand for IVD services, since more admissions mean more routine panels, screening tests, treatment-monitoring assays, and emergency diagnostics conducted per patient.
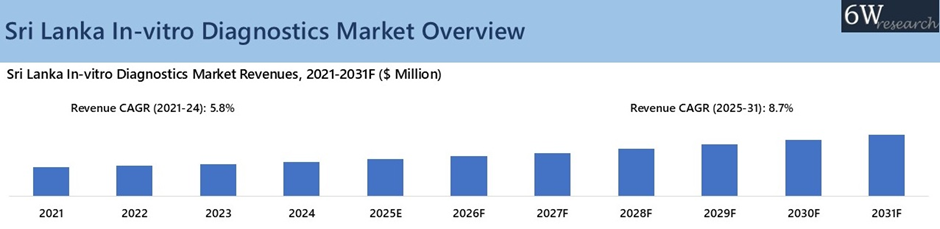
According to 6Wresearch, Sri Lanka In-vitro Diagnostics Market revenue size is projected to grow at a CAGR of 8.7% during 2025-2031. This expansion is reinforced by Sri Lanka’s plan to build a pharma manufacturing zone in the southern coast of Hambantota and Synergy Pharmaceuticals Corporation (Pvt) Ltd factory, within the Bingiriya Export Processing Zone, both under construction, once operational, these plants would run extensive biochemical, microbiology, sterility, and immunoassay quality-control lines, directly driving higher demand for analyzers, reagents, calibrators, and other IVD consumables. Rising chronic further accelerates IVD demand. Cancer cases are expected to increase by 2035, while diabetes would grow from 2024 to 2050.
These conditions require continuous diagnostics, driving more hematology, immunoassay, molecular, glucose, HbA1c, renal, and lipid testing, resulting in higher test volumes, heavier reagent use, and greater analyzer throughput across hospitals and private labs. Additionally, Sri Lanka’s rapid shift toward an ageing population, citizens aged 60+ by 2040—would intensify NCD prevalence, including diabetes, CKD, cardiovascular disease, thyroid disorders, and cancers. These conditions rely on routine biochemical, immunoassay, hematology, and molecular testing, pushing test volumes higher and increasing demand for analyzers, reagents, calibrators, and point-of-care systems across public facilities, thereby driving sustained market expansion in the coming years.
Market Segmentation By Application
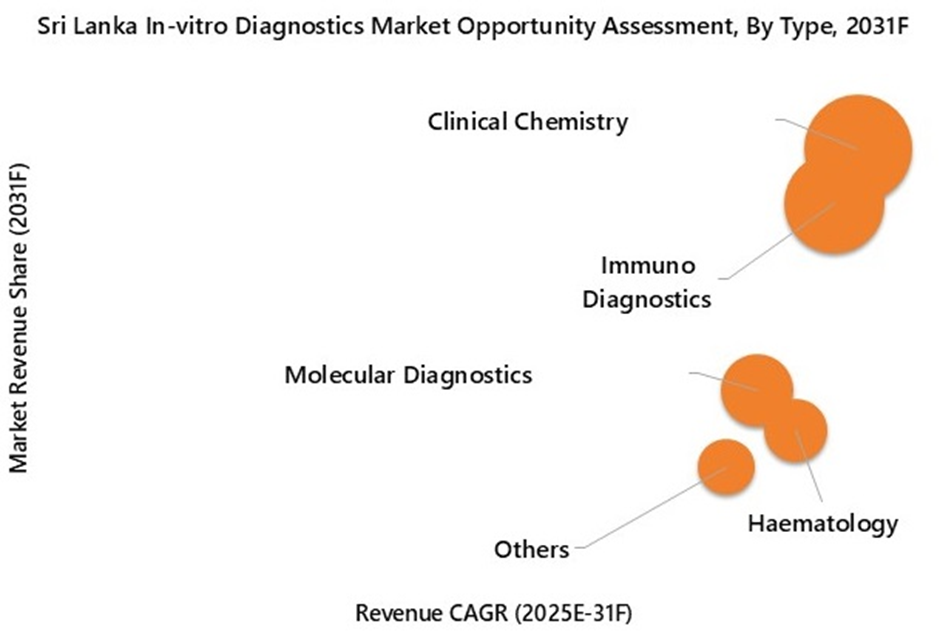
Cancer diagnostics is expected to record the highest growth rate in Sri Lanka’s In-Vitro Diagnostics Market by 2031, driven by a rapidly expanding patient pool and a currently small testing base, which creates significant incremental demand. Cancer incidence is projected to rise sharply by 2035. This growth will directly boost the utilization of tumor markers, oncology immunoassays, HPV screening, and biopsy-linked pathology workflows.
Market Segmentation By Product
The reagents & kits segment in Sri Lanka In-Vitro Diagnostics Market is expected to witness the fastest growth from 2025 to 2031, driven by rising test frequency across public and private labs where diagnostics depend on continuous reagent supply rather than one-time analyzer purchases. Sri Lanka’s heavy burden of NCDs, accounting for total deaths, pushes routine high-repeat testing for glucose, HbA1c, lipid profiles and cardiac panels, creating sustained reagent demand.
Market Segmentation By End-User
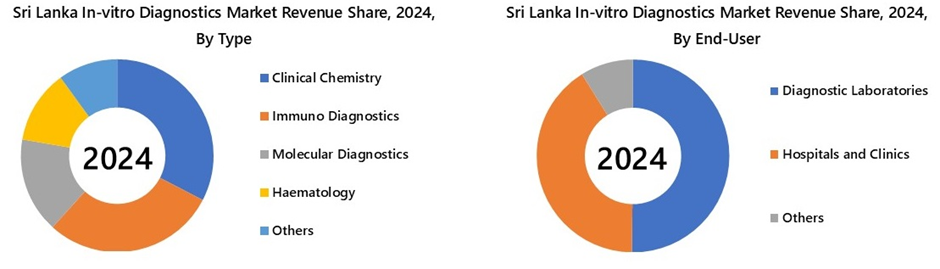
The diagnostic laboratories segment is expected to witness the highest growth rate in Sri Lanka’s In-Vitro Diagnostics Market by 2031 due to expanding test volumes, increasing outsourcing of complex assays, and broader healthcare access. Rising prevalence of NCDs and cancer drives higher demand for routine and specialized testing, while public health initiatives and private hospital networks push for centralized testing through high-throughput labs rather than smaller point-of-care setups.
Market Segmentation By Type
Clinical chemistry is projected to experience the highest growth rate in Sri Lanka’s In-Vitro Diagnostics Market by 2031, driven by the country’s growing non-communicable disease (NCD) burden. Routine tests like glucose, HbA1c, lipid profiles, and renal and liver function tests are essential for monitoring diabetes, cardiovascular conditions, and chronic kidney disease (CKD), which account for over three-quarters of Sri Lanka's disease burden. As screening programs expand and private diagnostic chains increase collection points, the volume of these baseline tests is expected to grow much faster than specialty segments.
Key Attractiveness of the Report
- 10 Years Market Numbers.
- Historical Data Starting from 2021 to 2024
- Base Year: 2024
- Forecast Data until 2031
- Key Performance Indicators Impacting the Market.
- Major Upcoming Developments and Projects.
Key Highlights of the Report:
- Global In-vitro Diagnostics Market Overview
- Sri Lanka In-vitro Diagnostics Market Overview
- Sri Lanka In-vitro Diagnostics Market Outlook
- Sri Lanka In-vitro Diagnostics Market Forecast
- Historical Data and Forecast of Sri Lanka In-vitro Diagnostics Market Revenues for the Period 2021-2031F
- Historical Data and Forecast of Sri Lanka In-vitro Diagnostics Market Revenues, By Product, for the Period 2021-2031F
- Historical Data and Forecast of Sri Lanka In-vitro Diagnostics Market Revenues, By Type, for the Period 2021-2031F
- Historical Data and Forecast of Sri Lanka In-vitro Diagnostics Market Revenues, By Application, for the Period 2021-2031F
- Historical Data and Forecast of Sri Lanka In-vitro Diagnostics Market Revenues, By End-User, for the Period 2021-2031F
- Industry Life Cycle
- Porter’s Five Force Analysis
- Sri Lanka In-vitro Diagnostics Market Drivers and Restraints
- Market Trends & Evolution
- Market Opportunity Assessment
- Sri Lanka In-vitro Diagnostics Market Revenue Ranking, By Top 3 Companies
- Competitive Benchmarking
- Company Profiles
- Key Strategic Recommendations
Market Scope and Segmentation
The report provides a detailed analysis of the following market segments:
By Product
- Instruments
- Reagents & kits
- Software & Services
By Type
- Clinical Chemistry
- Molecular Diagnostics
- Immuno Diagnostics
- Haematology
- Others (Urinalysis, Coagulation etc.)
By Application
- Infectious Disease
- Diabetes
- Cancer/Oncology
- Cardiology
- Autoimmune Disease
- Nephrology
- Others (Pulmonology, Gastroenterology, Haematology etc,.)
By End-User
- Diagnostic Laboratories
- Hospitals and Clinics
- Others (Pharmacies, Public health centers etc.)
Sri Lanka In-Vitro Diagnostics Market (2025-2031): FAQs
| 1. Executive Summary |
| 2. Introduction |
| 2.1. Report Description |
| 2.2. Key Highlights of the Report |
| 2.3. Market Scope & Segmentation |
| 2.4. Research Methodology |
| 2.5. Assumptions |
| 3. Global In-vitro Diagnostics Market Overview |
| 3.1. Global In-vitro Diagnostics Market Revenues, 2021-2031F |
| 4. Sri Lanka In-vitro Diagnostics Market Overview |
| 4.1. Sri Lanka Macroeconomic Indicators |
| 4.2. Sri Lanka In-vitro Diagnostics Market Revenues, 2021-2031F |
| 4.3. Sri Lanka In-vitro Diagnostics Market Industry Life Cycle |
| 4.4. Sri Lanka In-vitro Diagnostics Market Porter's Five Forces |
| 5. Sri Lanka In-vitro Diagnostics Market Dynamics |
| 5.1. Impact Analysis |
| 5.2. Market Drivers |
| 5.3. Market Restraints |
| 6. Sri Lanka In-vitro Diagnostics Market Trends |
| 7. Sri Lanka In-vitro Diagnostics Market Overview, By Product |
| 7.1. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Product, 2024 & 2031F |
| 7.1.1. Sri Lanka In-vitro Diagnostics Market Revenues, By Instruments, 2021-2031F |
| 7.1.2. Sri Lanka In-vitro Diagnostics Market Revenues, By Reagents & kits, 2021-2031F |
| 7.1.3. Sri Lanka In-vitro Diagnostics Market Revenues, By Software & Services, 2021-2031F |
| 8. Sri Lanka In-vitro Diagnostics Market Overview, By Type |
| 8.1. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Type, 2024 & 2031F |
| 8.1.1. Sri Lanka In-vitro Diagnostics Market Revenues, By Clinical Chemistry, 2021-2031F |
| 8.1.2. Sri Lanka In-vitro Diagnostics Market Revenues, By Molecular Diagnostics, 2021-2031F |
| 8.1.3. Sri Lanka In-vitro Diagnostics Market Revenues, By Immuno Diagnostics, 2021-2031F |
| 8.1.4. Sri Lanka In-vitro Diagnostics Market Revenues, By Haematology, 2021-2031F |
| 8.1.5. Sri Lanka In-vitro Diagnostics Market Revenues, By Others, 2021-2031F |
| 9. Sri Lanka In-vitro Diagnostics Market Overview, By Application |
| 9.1. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Application, 2024 & 2031F |
| 9.1.1. Sri Lanka In-vitro Diagnostics Market Revenues, By Infectious Disease, 2021-2031F |
| 9.1.2. Sri Lanka In-vitro Diagnostics Market Revenues, By Diabetes, 2021-2031F |
| 9.1.3. Sri Lanka In-vitro Diagnostics Market Revenues, By Cancer/Oncology, 2021-2031F |
| 9.1.4. Sri Lanka In-vitro Diagnostics Market Revenues, By Cardiology, 2021-2031F |
| 9.1.5. Sri Lanka In-vitro Diagnostics Market Revenues, By Autoimmune Disease, 2021-2031F |
| 9.1.6. Sri Lanka In-vitro Diagnostics Market Revenues, By Nephrology, 2021-2031F |
| 9.1.7. Sri Lanka In-vitro Diagnostics Market Revenues, By Others, 2021-2031F |
| 10. Sri Lanka In-vitro Diagnostics Market Overview, By End-User |
| 10.1. Sri Lanka In-vitro Diagnostics Market Revenue Share, By End-User, 2024 & 2031F |
| 10.1.1. Sri Lanka In-vitro Diagnostics Market Revenues, By Diagnostic Laboratories, 2021-2031F |
| 10.1.2. Sri Lanka In-vitro Diagnostics Market Revenues, By Hospitals and Clinics, 2021-2031F |
| 10.1.3. Sri Lanka In-vitro Diagnostics Market Revenues, By Others, 2021-2031F |
| 11. Sri Lanka In-vitro Diagnostics Market Key Performance Indicators |
| 12. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment |
| 12.1. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Product, 2031F |
| 12.2. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Type, 2031F |
| 12.3. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Application, 2031F |
| 12.4. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By End-User, 2031F |
| 13. Sri Lanka In-vitro Diagnostics Market Competitive Landscape |
| 13.1. Sri Lanka In-vitro Diagnostics Market Revenue Ranking, By Top 3 Companies, 2024 |
| 13.2. Sri Lanka In-vitro Diagnostics Market Competitive Benchmarking, By Technical Parameters |
| 13.3. Sri Lanka In-vitro Diagnostics Market Competitive Benchmarking, By Operating Parameters |
| 14. Company Profiles |
| 14.1 Siemens Healthineers AG |
| 14.2 Sysmex Corporation |
| 14.3 Agilent Technologies, Inc. |
| 14.4 Illumina, Inc. |
| 14.5 Abbott |
| 14.6 Tulip Diagnostics (P) Ltd. |
| 14.7 HUMAN Gesellschaft für Biochemica und Diagnostica mbH |
| 14.8 Shenzhen Mindray Bio-Medical Electronics Co. |
| 14.9 TaiDoc Technology Corporation |
| 14.10 Roche Diagnostics Asia Pacific Pte Ltd |
| 15. Key Strategic Recommendations |
| 16. Disclaimer |
| List of Figures |
| 1. Global In-vitro Diagnostics Market Revenues, 2021-2031F ($ Billion) |
| 2. Sri Lanka GDP, 2021-2024, (In $ Billion)) |
| 3. Sri Lanka GDP per Capita, 2021-2024, (In $) |
| 4. Sri Lanka Total Population, 2020-2040F, (In Million) |
| 5. Sri Lanka In-vitro Diagnostics Market Revenues, 2021-2031F ($ Million) |
| 6. Sri Lanka Number of Dengue Cases (2022-2023) |
| 7. Sri Lanka Number Of Microscopically Confirmed Malaria Cases (2022-2023) |
| 8. Number of Admissions Due to Selected NCDs (Non-Communicable Diseases) In Government Hospitals, 2021-2023 |
| 9. Sri Lanka Number Of Adults (20–79 Years) With Diabetes, (in Million), (2024-2050F) |
| 10. Sri Lanka Cancer Incidence, 2025E-30F, (in Thousands), 2022-2035F |
| 11. Sri Lanka Number Of Tuberculosis Cases (2021-2023) |
| 12. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Product, 2024 & 2031F |
| 13. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Type, 2024 & 2031F |
| 14. Sri Lanka In-vitro Diagnostics Market Revenue Share, By Application, 2024 & 2031F |
| 15. Sri Lanka In-vitro Diagnostics Market Revenue Share, By End-User, 2024 & 2031F |
| 16. Sri Lanka Healthcare Sector Overview |
| 17. Sri Lanka’s Government Health Expenditure In Public Health Sector, 2021-2023 ($ Billion) |
| 18. Sri Lanka Per Capita Health Expenditure In Public Health Sector 2021 - 2023 ($) |
| 19. Sri Lanka Number Of Hospitalizations By Cause Of Hospitalization, 2022-2023 |
| 20. Sri Lanka Pharmaceutical Market, in Million (2024-2025) |
| 21. Sri Lanka Manufacture of Pharmaceutical Products Sector Contribution to the GDP at Current Market Price (in INR Million), 2020-2021 |
| 22. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Product, 2031F |
| 23. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Type, 2031F |
| 24. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By Application, 2031F |
| 25. Sri Lanka In-vitro Diagnostics Market Opportunity Assessment, By End-User, 2031F |
| 26. Sri Lanka In-vitro Diagnostics Market Revenue Ranking, By Companies, CY2024 |
| List of tables |
| 1. Sri Lanka In-vitro Diagnostics Market Revenues, By Product, 2021-2031F ($ Million) |
| 2. Sri Lanka In-vitro Diagnostics Market Revenues, By Type, 2021-2031F ($ Million) |
| 3. Sri Lanka In-vitro Diagnostics Market Revenues, By Application, 2021-2031F ($ Million) |
| 4. Sri Lanka In-vitro Diagnostics Market Revenues, By End-User, 2021-2031F ($ Million) |
| 5. Sri Lanka Pharmaceutical Manufacturing Facilities |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















