China Neurology Devices Market Outlook | Revenue, Industry, Size, Forecast, Analysis, Companies, Value, Trends, Growth, COVID-19 IMPACT & Share
| Product Code: ETC367641 | Publication Date: Aug 2022 | Updated Date: Apr 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
China Neurology Devices Market Size Growth Rate
The China Neurology Devices Market may undergo a gradual slowdown in growth rates between 2025 and 2029. Beginning strongly at 8.71% in 2025, growth softens to 6.67% in 2029.
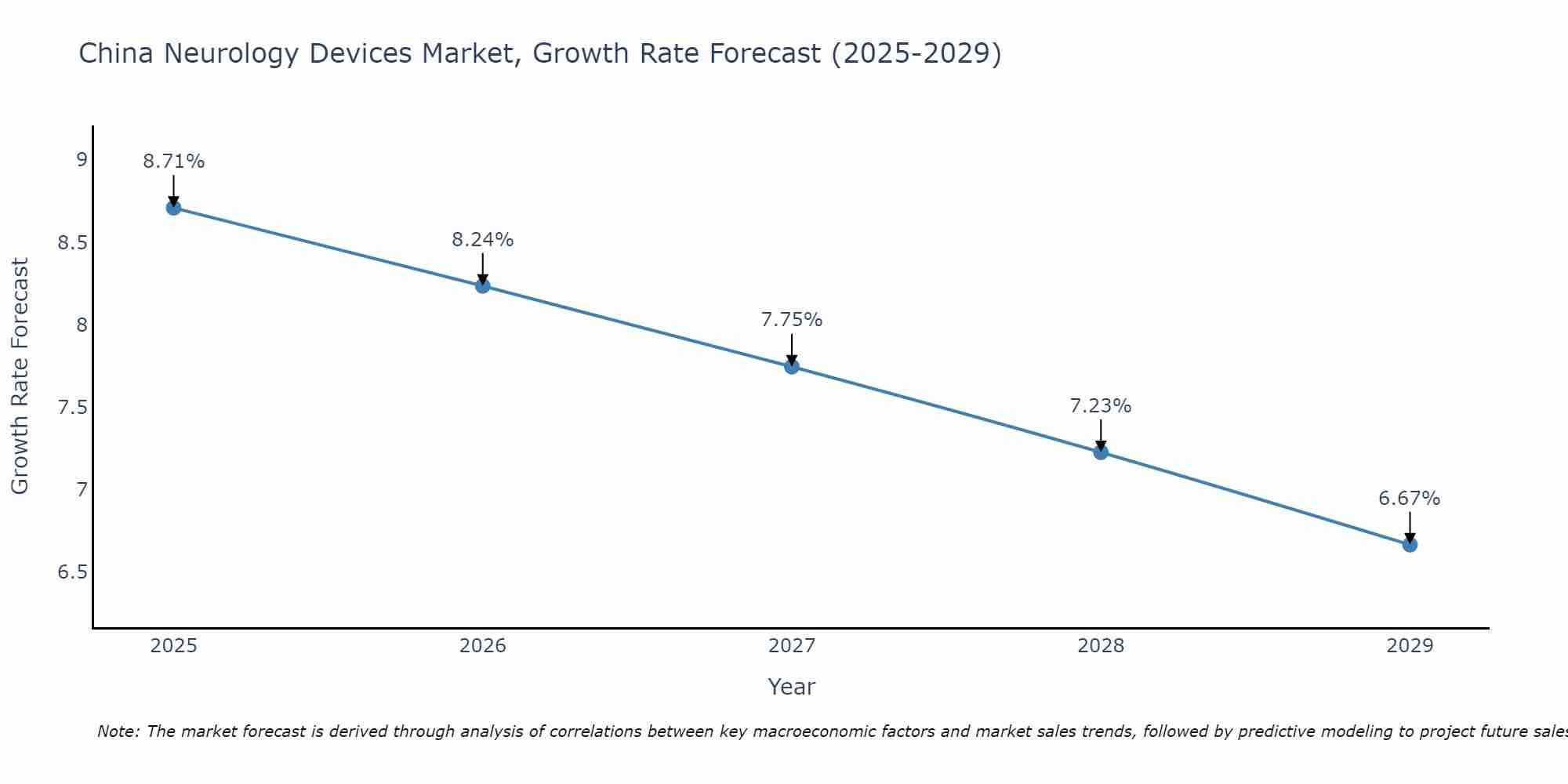
China Neurology Devices Market Overview
The Neurology Devices market in China is expanding as the demand for advanced medical technologies for diagnosing and treating neurological disorders grows. Devices in this sector include EEG machines, neurostimulators, and imaging systems, which are crucial for managing conditions such as epilepsy, Parkinson`s disease, and multiple sclerosis. The market is driven by an aging population, increasing prevalence of neurological conditions, and advancements in medical technology. Chinese healthcare providers and medical device manufacturers are focusing on developing and adopting innovative neurology devices to improve patient care and outcomes.
Drivers of the market
The Neurology Devices market in China is influenced by the demand for medical devices used in the diagnosis and treatment of neurological disorders. Neurology devices offer benefits such as improved diagnostic accuracy, effective treatment options, and enhanced patient care. Market growth is driven by advancements in medical technologies, increasing prevalence of neurological conditions, and the need for innovative diagnostic and therapeutic solutions.
Challenges of the market
The China Neurology Devices Market encounters challenges related to the high cost of developing and manufacturing advanced neurology devices. Moreover, the market must navigate regulatory requirements related to the safety and efficacy of neurology devices.
Government Policy of the market
Chinas neurology devices market is influenced by government regulations focused on medical technology and patient safety. The government establishes standards for the performance and safety of neurology devices to ensure they meet industry requirements. Policies support the development and adoption of advanced medical technologies through funding and research incentives. The government also addresses concerns related to medical device safety and promotes best practices in neurology device manufacturing.
Key Highlights of the Report:
- China Neurology Devices Market Outlook
- Market Size of China Neurology Devices Market, 2024
- Forecast of China Neurology Devices Market, 2031
- Historical Data and Forecast of China Neurology Devices Revenues & Volume for the Period 2018 - 2031
- China Neurology Devices Market Trend Evolution
- China Neurology Devices Market Drivers and Challenges
- China Neurology Devices Price Trends
- China Neurology Devices Porter's Five Forces
- China Neurology Devices Industry Life Cycle
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Product for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Neurostimulation Devices for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Neurosurgery Devices for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Interventional Neurology Devices for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Cerebrospinal fluid management devices for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Others for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By End User for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Hospitals for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Ambulatory surgery centers for the Period 2018 - 2031
- Historical Data and Forecast of China Neurology Devices Market Revenues & Volume By Neurology clinics for the Period 2018 - 2031
- China Neurology Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By End User
- China Neurology Devices Top Companies Market Share
- China Neurology Devices Competitive Benchmarking By Technical and Operational Parameters
- China Neurology Devices Company Profiles
- China Neurology Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Uganda Precast and Aggregate Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Australia IT Asset Disposal Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- UAE Building Thermal Insulation Market Outlook (2025-2031) | Revenue, Companies, Share, Trends, Growth, Size, Forecast, Industry, Analysis & Value
- Portugal Electronic Document Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













