France Primary Cells Market Outlook | Trends, Analysis, Companies, Revenue, Forecast, Industry, COVID-19 IMPACT, Share, Size, Value & Growth
| Product Code: ETC269110 | Publication Date: Aug 2022 | Updated Date: Aug 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sachin Kumar Rai | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
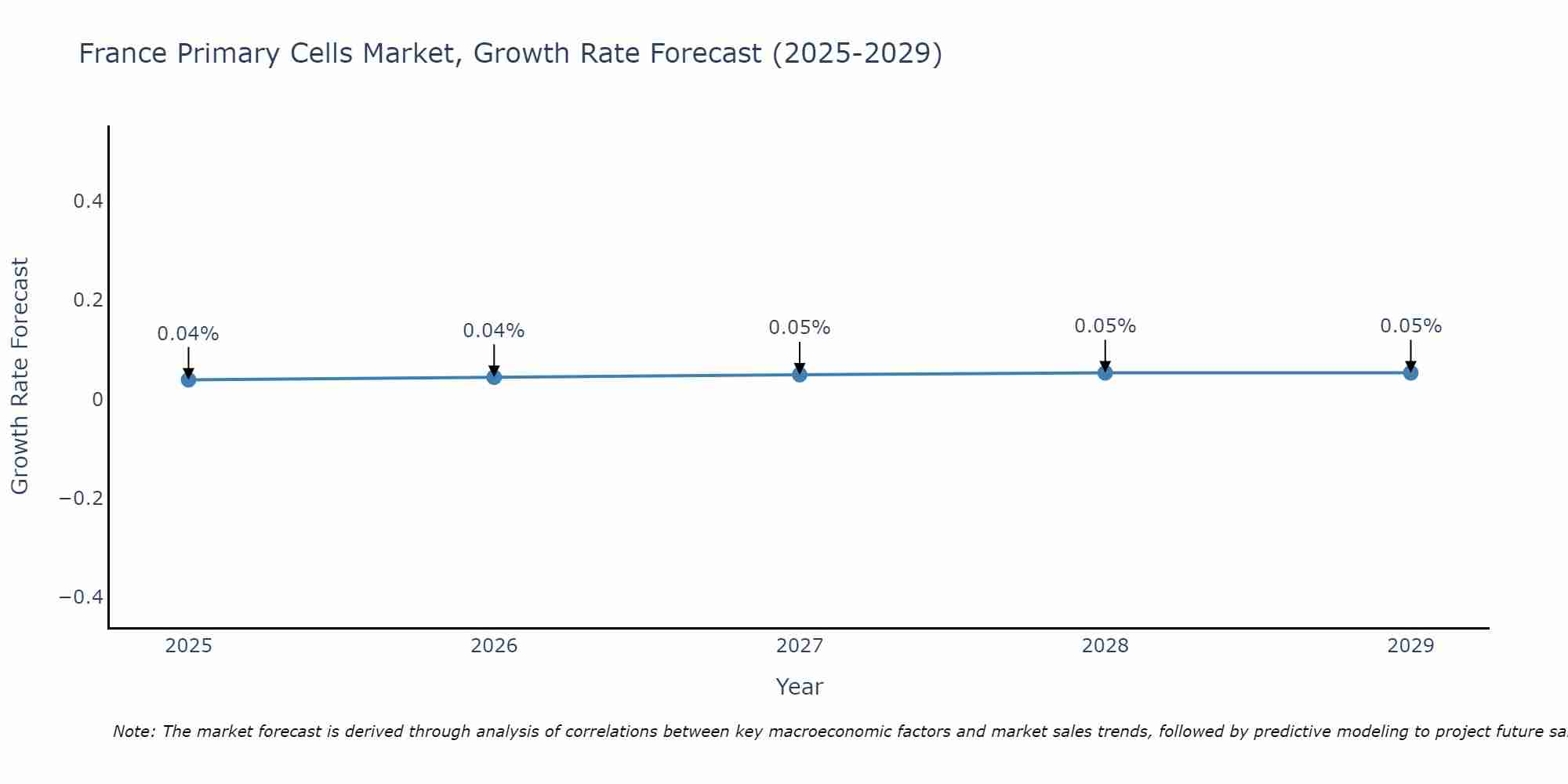
France Primary Cells Market Size Growth Rate
The France Primary Cells Market is poised for steady growth rate improvements from 2025 to 2029. From 0.04% in 2025, the growth rate steadily ascends to 0.05% in 2029.
France Primary Cells Market Overview
The France Primary Cells Market is experiencing steady growth driven by the increasing demand for primary cells in research and development activities across various industries such as pharmaceuticals, biotechnology, and academic research. Primary cells are essential tools for studying cell behavior, disease mechanisms, drug efficacy, and toxicity testing. The market is characterized by a wide range of primary cell types, including stem cells, immune cells, epithelial cells, and others, catering to diverse research needs. Key players in the France Primary Cells Market are focusing on expanding their product portfolios, improving cell quality, and establishing strategic partnerships to enhance their market presence. Factors such as technological advancements in cell culture techniques and the rising investments in life sciences research are expected to further drive the growth of the France Primary Cells Market in the coming years.
France Primary Cells Market Trends
The France Primary Cells Market is witnessing several key trends currently. One prominent trend is the increasing demand for personalized medicine, driving the growth of primary cell cultures for patient-specific research and drug development. Another significant trend is the rising adoption of 3D cell culture models using primary cells, enabling more accurate representation of in vivo conditions for drug screening and toxicity testing. Additionally, there is a growing focus on ethical sourcing of primary cells, with researchers prioritizing suppliers that adhere to ethical guidelines and animal welfare standards. Moreover, advancements in biobanking technologies and cryopreservation methods are enhancing the storage and preservation of primary cells for long-term use, supporting research efforts in various fields such as oncology, neuroscience, and regenerative medicine in France.
France Primary Cells Market Challenges
In the France Primary Cells Market, some key challenges include the high cost associated with primary cell culture maintenance and production, limited availability of specific cell types, ethical considerations surrounding the sourcing of primary cells from human or animal tissues, and variability in cell quality and performance. Additionally, there are regulatory hurdles related to the use of primary cells in research and drug development, such as compliance with stringent guidelines for cell authentication, quality control, and documentation. Moreover, the need for specialized skills and expertise in primary cell culture techniques further complicates the process of working with primary cells in the French market. Overall, addressing these challenges requires strategic investments in research and development, collaboration with reliable cell suppliers, and adherence to strict regulatory requirements to ensure the reliability and reproducibility of primary cell-based research in France.
France Primary Cells Market Investment Opportunities
The France Primary Cells Market offers promising investment opportunities, driven by the increasing demand for primary cells in research and development activities across various industries such as pharmaceuticals, biotechnology, and academic research. With the growing focus on personalized medicine and regenerative therapies, there is a rising need for high-quality primary cells for drug discovery, toxicology testing, and disease modeling. Investing in companies that specialize in the production and supply of primary cells, as well as those offering related services such as cell isolation and customization, could be lucrative. Additionally, advancements in technology and the emergence of innovative cell culture techniques provide further growth potential in this market. Overall, the France Primary Cells Market presents a fertile ground for investors looking to capitalize on the expanding biotechnology and life sciences sectors.
France Primary Cells Market Government Policy
In France, the primary cells market is subject to regulatory policies overseen by agencies such as the French National Agency for Medicines and Health Products Safety (ANSM) and the Ministry of Health. These policies focus on ensuring the safety, quality, and efficacy of primary cells used in research and therapeutic applications. Companies operating in this market must comply with strict regulations regarding cell sourcing, processing, storage, and distribution to protect patient safety and maintain product integrity. Additionally, ethical considerations, such as obtaining informed consent for cell donation and respecting donor rights, are key aspects of the regulatory framework governing the primary cells market in France. Adherence to these government policies is essential for market participants to maintain compliance and uphold ethical standards in the development and use of primary cells.
France Primary Cells Market Future Outlook
The France Primary Cells Market is expected to witness steady growth in the coming years due to the increasing demand for cell-based research and therapies. Factors such as the rising prevalence of chronic diseases, advancements in cell culture technologies, and a growing focus on personalized medicine are driving the market expansion. Additionally, the increasing investments in research and development activities by biotechnology and pharmaceutical companies are expected to further propel market growth. However, challenges such as stringent regulations and ethical concerns related to cell sourcing may hinder the market growth to some extent. Overall, the France Primary Cells Market is anticipated to experience sustained growth in the foreseeable future, with opportunities for innovation and expansion in various applications including drug discovery, regenerative medicine, and cancer research.
Key Highlights of the Report:
- France Primary Cells Market Outlook
- Market Size of France Primary Cells Market, 2021
- Forecast of France Primary Cells Market, 2031
- Historical Data and Forecast of France Primary Cells Revenues & Volume for the Period 2018 - 2031
- France Primary Cells Market Trend Evolution
- France Primary Cells Market Drivers and Challenges
- France Primary Cells Price Trends
- France Primary Cells Porter's Five Forces
- France Primary Cells Industry Life Cycle
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Origin for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Human Primary Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Animal Primary Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Type for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Hematopoietic Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Dermatocytes for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Gastrointestinal Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Hepatocytes for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Lung Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Renal Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Heart Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Musculoskeletal Cells for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By End User for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Life Science Research Companies for the Period 2018 - 2031
- Historical Data and Forecast of France Primary Cells Market Revenues & Volume By Research Institutes for the Period 2018 - 2031
- France Primary Cells Import Export Trade Statistics
- Market Opportunity Assessment By Origin
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By End User
- France Primary Cells Top Companies Market Share
- France Primary Cells Competitive Benchmarking By Technical and Operational Parameters
- France Primary Cells Company Profiles
- France Primary Cells Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Primary Cells Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Primary Cells Market Revenues & Volume, 2021 & 2031F |
3.3 France Primary Cells Market - Industry Life Cycle |
3.4 France Primary Cells Market - Porter's Five Forces |
3.5 France Primary Cells Market Revenues & Volume Share, By Origin, 2021 & 2031F |
3.6 France Primary Cells Market Revenues & Volume Share, By Type, 2021 & 2031F |
3.7 France Primary Cells Market Revenues & Volume Share, By End User, 2021 & 2031F |
4 France Primary Cells Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing demand for personalized medicine and regenerative therapies |
4.2.2 Growing research and development activities in the field of biotechnology and healthcare |
4.2.3 Rising prevalence of chronic diseases leading to a higher demand for primary cells |
4.3 Market Restraints |
4.3.1 Stringent regulations and ethical considerations related to the use of primary cells |
4.3.2 High costs associated with primary cell culture and maintenance |
4.3.3 Limited availability of specialized primary cells for research purposes |
5 France Primary Cells Market Trends |
6 France Primary Cells Market, By Types |
6.1 France Primary Cells Market, By Origin |
6.1.1 Overview and Analysis |
6.1.2 France Primary Cells Market Revenues & Volume, By Origin, 2021-2031F |
6.1.3 France Primary Cells Market Revenues & Volume, By Human Primary Cells, 2021-2031F |
6.1.4 France Primary Cells Market Revenues & Volume, By Animal Primary Cells, 2021-2031F |
6.2 France Primary Cells Market, By Type |
6.2.1 Overview and Analysis |
6.2.2 France Primary Cells Market Revenues & Volume, By Hematopoietic Cells, 2021-2031F |
6.2.3 France Primary Cells Market Revenues & Volume, By Dermatocytes, 2021-2031F |
6.2.4 France Primary Cells Market Revenues & Volume, By Gastrointestinal Cells, 2021-2031F |
6.2.5 France Primary Cells Market Revenues & Volume, By Hepatocytes, 2021-2031F |
6.2.6 France Primary Cells Market Revenues & Volume, By Lung Cells, 2021-2031F |
6.2.7 France Primary Cells Market Revenues & Volume, By Renal Cells, 2021-2031F |
6.2.8 France Primary Cells Market Revenues & Volume, By Musculoskeletal Cells, 2021-2031F |
6.2.9 France Primary Cells Market Revenues & Volume, By Musculoskeletal Cells, 2021-2031F |
6.3 France Primary Cells Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 France Primary Cells Market Revenues & Volume, By Life Science Research Companies, 2021-2031F |
6.3.3 France Primary Cells Market Revenues & Volume, By Research Institutes, 2021-2031F |
7 France Primary Cells Market Import-Export Trade Statistics |
7.1 France Primary Cells Market Export to Major Countries |
7.2 France Primary Cells Market Imports from Major Countries |
8 France Primary Cells Market Key Performance Indicators |
8.1 Average time taken for primary cell culture establishment |
8.2 Percentage of successful primary cell cultures obtained |
8.3 Rate of adoption of advanced primary cell culture techniques |
9 France Primary Cells Market - Opportunity Assessment |
9.1 France Primary Cells Market Opportunity Assessment, By Origin, 2021 & 2031F |
9.2 France Primary Cells Market Opportunity Assessment, By Type, 2021 & 2031F |
9.3 France Primary Cells Market Opportunity Assessment, By End User, 2021 & 2031F |
10 France Primary Cells Market - Competitive Landscape |
10.1 France Primary Cells Market Revenue Share, By Companies, 2021 |
10.2 France Primary Cells Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Uganda Precast and Aggregate Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Australia IT Asset Disposal Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- UAE Building Thermal Insulation Market Outlook (2025-2031) | Revenue, Companies, Share, Trends, Growth, Size, Forecast, Industry, Analysis & Value
- Portugal Electronic Document Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













