Japan Biologics Market (2025-2031) | Revenue, Analysis, Value, Forecast, Share, Growth, Industry, Size, Outlook, Trends & Companies
| Product Code: ETC052442 | Publication Date: Jan 2021 | Updated Date: Oct 2025 | Product Type: Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 70 | No. of Figures: 35 | No. of Tables: 5 |
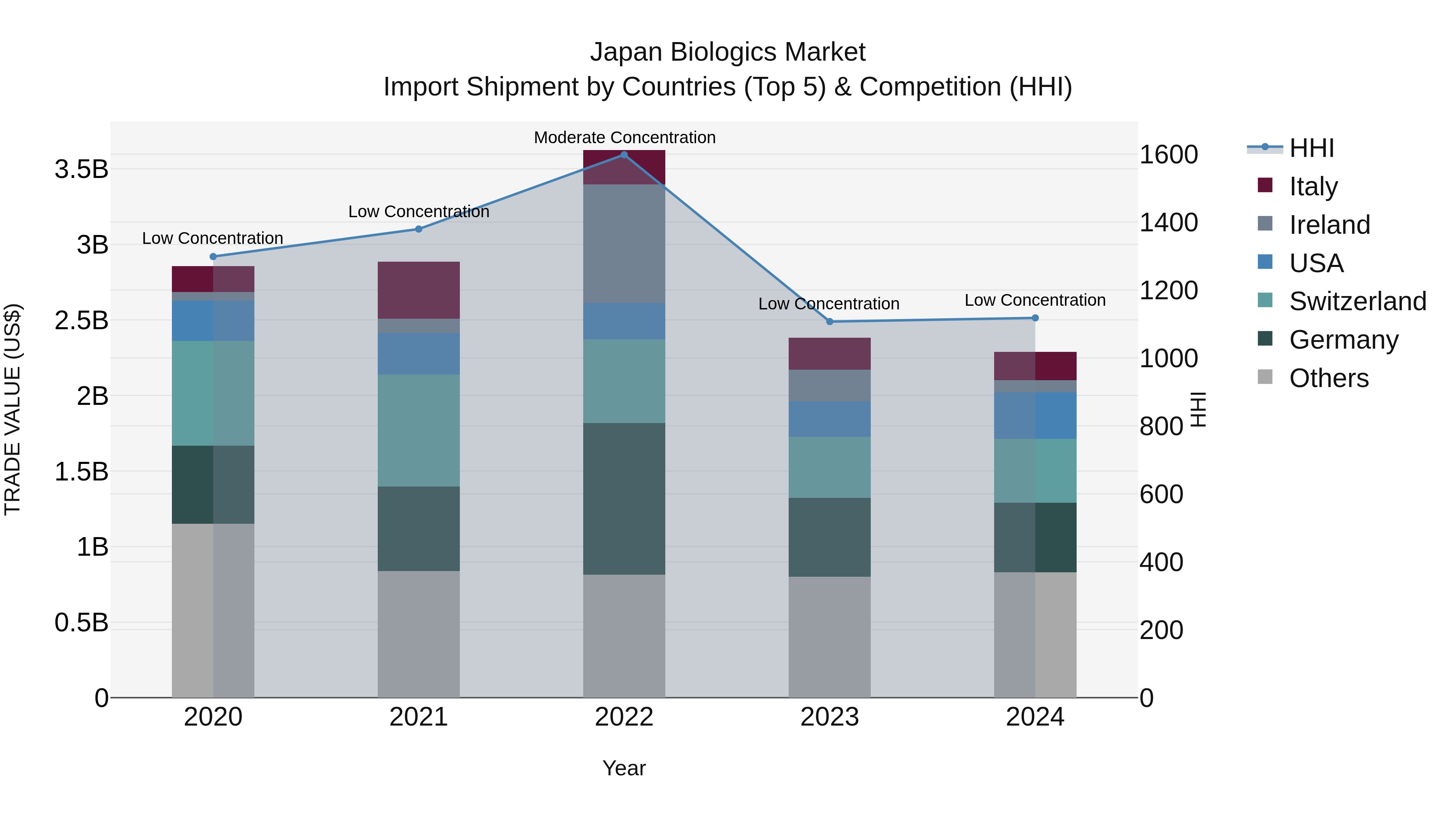
Japan Biologics Market Import Shipment by Countries (Top 5) & Competition (HHI)
Despite a declining Compound Annual Growth Rate (CAGR) and negative growth rate in 2024, Japan's biologics import market continues to be diverse with top exporters like Germany, Switzerland, USA, Italy, and Metropolitan France. The low Herfindahl-Hirschman Index (HHI) concentration indicates a competitive landscape, providing Japanese buyers with various options and ensuring a healthy market dynamic. Keeping an eye on these trends can help stakeholders navigate challenges and capitalize on opportunities in the evolving biologics import market in Japan.
Japan Biologics Market Overview
The Japan Biologics Market is a rapidly growing sector within the country`s pharmaceutical industry, driven by advancements in biotechnology and increasing demand for innovative biologic therapies. Biologics have gained popularity for their effectiveness in treating complex diseases like cancer, autoimmune disorders, and rare genetic conditions. The market is characterized by a strong regulatory framework ensuring product safety and quality standards, with major players investing in research and development to introduce new biologic drugs to cater to the evolving healthcare needs of the population. Factors such as an aging population, high healthcare expenditure, and government support for biopharmaceutical innovation are contributing to the expansion of the Japan Biologics Market, making it a key area of focus for industry stakeholders and investors.
Japan Biologics Market Trends
The Japan biologics market is experiencing significant growth driven by factors such as increasing demand for innovative therapies, rising prevalence of chronic diseases, and advancements in biotechnology. Biosimilars are gaining traction in Japan, offering cost-effective alternatives to expensive biologic drugs. The government`s initiatives to promote the use of biosimilars and expedite the approval process are further fueling market expansion. Collaborations between domestic and international biopharmaceutical companies are on the rise, fostering research and development activities in the country. Additionally, the aging population and changing healthcare landscape are driving the adoption of personalized medicine and targeted biologic therapies in Japan. Overall, the Japan biologics market is poised for continued growth and innovation in the coming years.
Japan Biologics Market Challenges
In the Japan biologics market, challenges include stringent regulatory requirements, high production costs, and competition from biosimilars. Regulatory approvals for biologics are complex and time-consuming, leading to delays in market entry. The high cost of developing and manufacturing biologics poses a barrier for smaller companies and startups. Additionally, the increasing availability of biosimilars, which are lower-cost alternatives to biologics, creates pricing pressure and competition within the market. Companies in Japan`s biologics sector must navigate these challenges by investing in research and development to differentiate their products, ensuring compliance with regulations, and optimizing manufacturing processes to reduce costs and improve competitiveness.
Japan Biologics Market Investment Opportunities
The Japan Biologics Market presents promising investment opportunities due to the increasing demand for biopharmaceutical products and advancements in biotechnology research. With a growing aging population and rising healthcare expenditure, there is a high demand for innovative biologics to treat chronic diseases such as cancer, autoimmune disorders, and rare genetic conditions. Companies involved in the development and commercialization of biologics, including biopharmaceutical manufacturers, contract research organizations, and biotech startups, are well-positioned to capitalize on this market growth. Additionally, collaborations between global pharmaceutical companies and Japanese biotech firms offer opportunities for partnerships and technology transfer. Investors looking to tap into the Japan Biologics Market can consider investing in biotech companies with a strong pipeline of biologics, as well as those focused on research and development in this rapidly evolving sector.
Japan Biologics Market Government Policy
Government policies in Japan related to the biologics market are aimed at promoting innovation, ensuring safety and efficacy of biologic products, and expanding access to advanced therapies. The Pharmaceuticals and Medical Devices Agency (PMDA) plays a key role in regulating biologics in Japan, overseeing the approval process and post-market surveillance. The government has implemented measures to streamline the regulatory pathway for biologics, such as expedited reviews for breakthrough therapies and conditional approvals based on preliminary data. Additionally, Japan has established a pricing system that balances the need for innovation with cost containment, including biennial price revisions for biologics. Overall, the government aims to foster a competitive and sustainable biologics market while prioritizing patient safety and access to cutting-edge therapies.
Japan Biologics Market Future Outlook
The Japan Biologics Market is poised for significant growth in the coming years due to increasing demand for biopharmaceutical products and advancements in biotechnology. Factors such as an aging population, rising prevalence of chronic diseases, and a strong regulatory environment supporting biologics development are driving market expansion. Additionally, collaborations between domestic and international biopharmaceutical companies, as well as investments in research and development, are likely to propel market growth further. The approval of biosimilars and innovative biologic therapies is expected to enhance market competition and provide patients with more treatment options. Overall, the Japan Biologics Market is set to experience robust growth and innovation, making it a key player in the global biopharmaceutical industry.
Key Highlights of the Report:
- Japan Biologics Market Outlook
- Market Size of Japan Biologics Market, 2024
- Forecast of Japan Biologics Market, 2031
- Historical Data and Forecast of Japan Biologics Revenues & Volume for the Period 2021 - 2031
- Japan Biologics Market Trend Evolution
- Japan Biologics Market Drivers and Challenges
- Japan Biologics Price Trends
- Japan Biologics Porter's Five Forces
- Japan Biologics Industry Life Cycle
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Source for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Microbial for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Mammalian for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Product Type for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Monoclonal Antibodies for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Vaccines for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Recombinant Proteins for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Antisense, RNAi, & Molecular Therapy for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Disease? Category for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Oncology for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Infectious Diseases for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Immunological Disorders for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Cardiovascular Disorders for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Hematological Disorders for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Biologics Manufacturing for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By Outsourced for the Period 2021 - 2031
- Historical Data and Forecast of Japan Biologics Market Revenues & Volume By In-house for the Period 2021 - 2031
- Japan Biologics Import Export Trade Statistics
- Market Opportunity Assessment By Source
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Disease? Category
- Market Opportunity Assessment By Biologics Manufacturing
- Japan Biologics Top Companies Market Share
- Japan Biologics Competitive Benchmarking By Technical and Operational Parameters
- Japan Biologics Company Profiles
- Japan Biologics Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Japan Biologics Market Overview |
3.1 Japan Country Macro Economic Indicators |
3.2 Japan Biologics Market Revenues & Volume, 2024 & 2031F |
3.3 Japan Biologics Market - Industry Life Cycle |
3.4 Japan Biologics Market - Porter's Five Forces |
3.5 Japan Biologics Market Revenues & Volume Share, By Source, 2021 & 2031F |
3.6 Japan Biologics Market Revenues & Volume Share, By Product Type, 2024 & 2031F |
3.7 Japan Biologics Market Revenues & Volume Share, By Disease Category, 2024 & 2031F |
3.8 Japan Biologics Market Revenues & Volume Share, By Biologics Manufacturing, 2024 & 2031F |
4 Japan Biologics Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing investment in research and development for biologics in Japan |
4.2.2 Growing prevalence of chronic diseases driving demand for biologics |
4.2.3 Favorable regulatory environment supporting the development and commercialization of biologics |
4.3 Market Restraints |
4.3.1 High cost associated with the development and production of biologics |
4.3.2 Stringent regulatory requirements for approval of biologics in Japan |
4.3.3 Limited availability of skilled professionals in biologics research and development |
5 Japan Biologics Market Trends |
6 Japan Biologics Market, By Types |
6.1 Japan Biologics Market, By Source |
6.1.1 Overview and Analysis |
6.1.2 Japan Biologics Market Revenues & Volume, By Source, 2016 - 2031F |
6.1.3 Japan Biologics Market Revenues & Volume, By Microbial, 2016 - 2031F |
6.1.4 Japan Biologics Market Revenues & Volume, By Mammalian, 2016 - 2031F |
6.1.5 Japan Biologics Market Revenues & Volume, By Others, 2016 - 2031F |
6.2 Japan Biologics Market, By Product Type |
6.2.1 Overview and Analysis |
6.2.2 Japan Biologics Market Revenues & Volume, By Monoclonal Antibodies, 2016 - 2031F |
6.2.3 Japan Biologics Market Revenues & Volume, By Vaccines, 2016 - 2031F |
6.2.4 Japan Biologics Market Revenues & Volume, By Recombinant Proteins, 2016 - 2031F |
6.2.5 Japan Biologics Market Revenues & Volume, By Antisense, RNAi, & Molecular Therapy, 2016 - 2031F |
6.2.6 Japan Biologics Market Revenues & Volume, By Others, 2016 - 2031F |
6.3 Japan Biologics Market, By Disease Category |
6.3.1 Overview and Analysis |
6.3.2 Japan Biologics Market Revenues & Volume, By Oncology, 2016 - 2031F |
6.3.3 Japan Biologics Market Revenues & Volume, By Infectious Diseases, 2016 - 2031F |
6.3.4 Japan Biologics Market Revenues & Volume, By Immunological Disorders, 2016 - 2031F |
6.3.5 Japan Biologics Market Revenues & Volume, By Cardiovascular Disorders, 2016 - 2031F |
6.3.6 Japan Biologics Market Revenues & Volume, By Hematological Disorders, 2016 - 2031F |
6.3.7 Japan Biologics Market Revenues & Volume, By Others, 2016 - 2031F |
6.4 Japan Biologics Market, By Biologics Manufacturing |
6.4.1 Overview and Analysis |
6.4.2 Japan Biologics Market Revenues & Volume, By Outsourced, 2016 - 2031F |
6.4.3 Japan Biologics Market Revenues & Volume, By In-house, 2016 - 2031F |
7 Japan Biologics Market Import-Export Trade Statistics |
7.1 Japan Biologics Market Export to Major Countries |
7.2 Japan Biologics Market Imports from Major Countries |
8 Japan Biologics Market Key Performance Indicators |
8.1 Number of new biologics in the pipeline for development and approval |
8.2 Research and development expenditure dedicated to biologics in Japan |
8.3 Number of collaborations and partnerships within the biologics industry in Japan |
9 Japan Biologics Market - Opportunity Assessment |
9.1 Japan Biologics Market Opportunity Assessment, By Source, 2024 & 2031F |
9.2 Japan Biologics Market Opportunity Assessment, By Product Type, 2024 & 2031F |
9.3 Japan Biologics Market Opportunity Assessment, By Disease Category, 2024 & 2031F |
9.4 Japan Biologics Market Opportunity Assessment, By Biologics Manufacturing, 2024 & 2031F |
10 Japan Biologics Market - Competitive Landscape |
10.1 Japan Biologics Market Revenue Share, By Companies, 2024 |
10.2 Japan Biologics Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Uganda Precast and Aggregate Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Australia IT Asset Disposal Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













