Netherlands Induced Pluripotent Stem Cells Market (2025-2031) | Analysis, Supply, Demand, Segmentation, Growth, Opportunities, Pricing Analysis, Strategy, Consumer Insights, Restraints, Drivers, Revenue, Strategic Insights, Industry, Companies, Segments, Share, Investment Trends, Outlook, Competition, Size, Competitive, Challenges, Trends, Value, Forecast
| Product Code: ETC12473484 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Deep | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
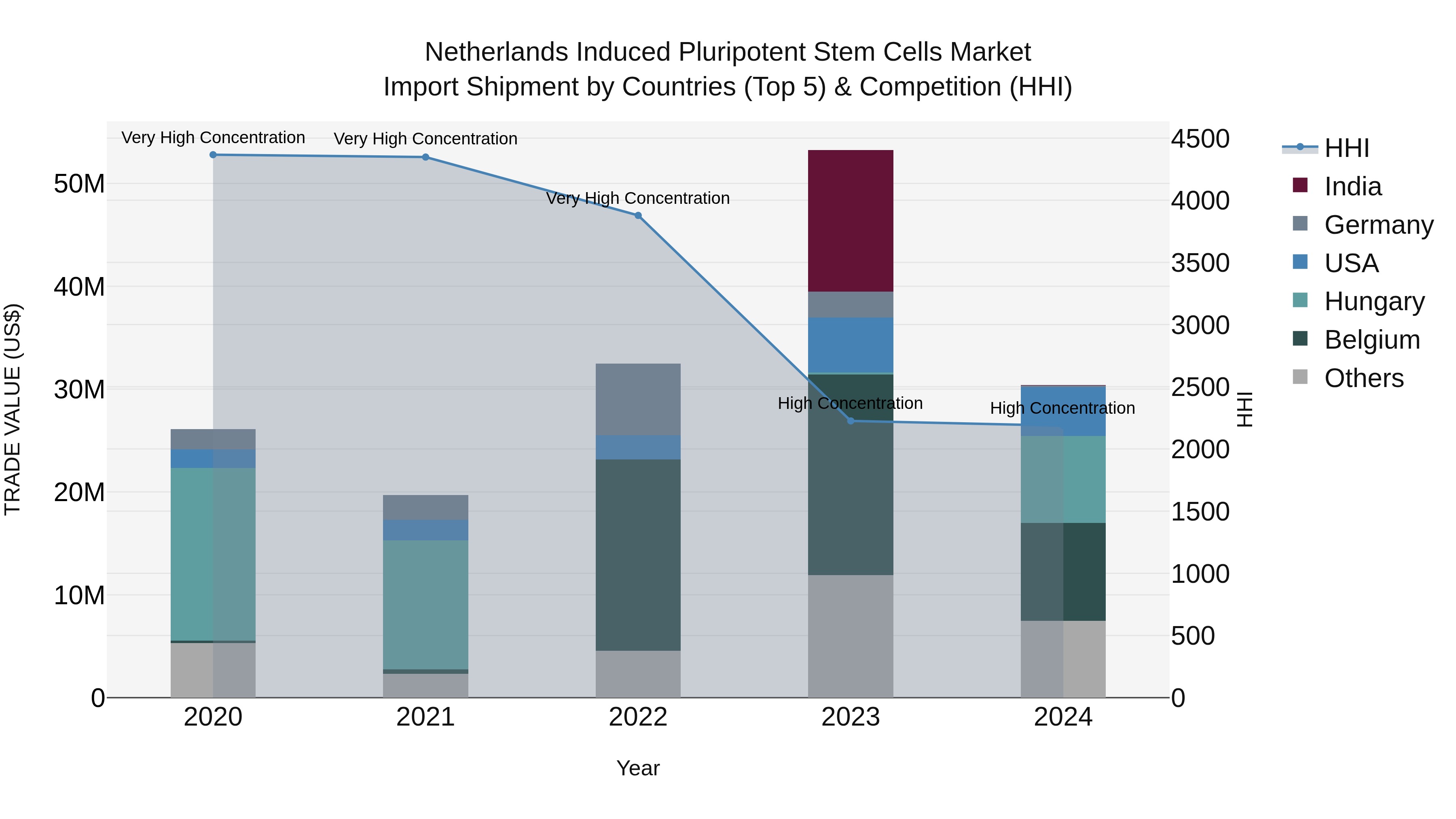
Netherlands Induced Pluripotent Stem Cells Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, the Netherlands continued to rely on imports of induced pluripotent stem cells, with top exporting countries including Belgium, Hungary, USA, Japan, and Sweden. The market remains highly concentrated, as indicated by the high Herfindahl-Hirschman Index (HHI). Despite a modest compound annual growth rate (CAGR) of 3.85% from 2020 to 2024, there was a significant decline in growth rate from 2023 to 2024 at -42.93%. This fluctuation suggests potential shifts in market dynamics or regulatory challenges impacting the import of stem cells in the Netherlands.
Netherlands Induced Pluripotent Stem Cells Market Market Overview
The Netherlands induced pluripotent stem cells market is experiencing steady growth driven by increasing investment in research and development, rising prevalence of chronic diseases, and growing adoption of regenerative medicine. Key players in the market are focused on collaborations and partnerships to enhance their research capabilities and expand their product offerings. The market is characterized by a strong regulatory framework ensuring quality and safety standards are met. Additionally, government initiatives promoting stem cell research and advancements in technology are contributing to market growth. The demand for induced pluripotent stem cells is expected to continue to rise in the Netherlands, driven by the potential applications in personalized medicine, drug discovery, and tissue engineering. Overall, the market presents significant opportunities for growth and innovation in the coming years.
Netherlands Induced Pluripotent Stem Cells Market Trends
In the Netherlands, the induced pluripotent stem cells (iPSC) market is witnessing several key trends. One significant trend is the increasing focus on personalized medicine and regenerative therapies, driving demand for iPSC technology in drug discovery and development. Collaborations between academic research institutions and biopharmaceutical companies are also on the rise, fostering innovation and the translation of iPSC research into clinical applications. Furthermore, there is a growing emphasis on automation and standardization in iPSC production processes to improve efficiency and scalability. The Netherlands` strong research infrastructure and supportive regulatory environment are further contributing to the growth of the iPSC market in the country, attracting investments and fostering advancements in this cutting-edge field.
Netherlands Induced Pluripotent Stem Cells Market Challenges
In the Netherlands, the induced pluripotent stem cells market faces challenges related to regulatory complexities, ethical considerations, and funding constraints. Regulatory frameworks governing the use of stem cells can be stringent and may vary across different regions, leading to compliance issues for researchers and companies. Ethical concerns surrounding the source of stem cells, particularly in the context of embryonic stem cells, can also impact research and development efforts. Additionally, limited funding opportunities for stem cell research projects can hinder innovation and the commercialization of new therapies. Addressing these challenges will require collaboration between researchers, industry stakeholders, and policymakers to create a supportive environment for the growth of the induced pluripotent stem cells market in the Netherlands.
Netherlands Induced Pluripotent Stem Cells Market Investment Opportunities
In the Netherlands, the induced pluripotent stem cells market presents promising investment opportunities due to the country`s strong research infrastructure and supportive regulatory environment for biotechnology and healthcare innovations. With a growing focus on regenerative medicine and personalized healthcare, there is increasing demand for advanced therapies utilizing induced pluripotent stem cells. Investing in companies or research institutions at the forefront of developing IPSC-based therapies and technologies could yield significant returns. Additionally, collaborations between academia, industry, and government initiatives further enhance the growth potential of the IPSC market in the Netherlands. Potential investment avenues include biotech startups, research partnerships, and technology platforms that facilitate the efficient and ethical use of induced pluripotent stem cells for therapeutic applications.
Netherlands Induced Pluripotent Stem Cells Market Government Policy
In the Netherlands, government policies regarding induced pluripotent stem cells (iPSCs) are focused on promoting research while ensuring ethical standards and patient safety. The government has established guidelines that regulate the use of iPSCs in research and clinical applications, including obtaining informed consent from donors, maintaining privacy and data protection, and monitoring the quality and safety of iPSC-derived products. Additionally, the Dutch government provides funding and support for iPSC research projects, aiming to advance scientific knowledge and innovation in regenerative medicine and drug development. Overall, the Netherlands has a favorable regulatory environment for iPSC research, with a strong emphasis on ethical considerations and quality control measures to foster advancements in the field while safeguarding the well-being of patients and donors.
Netherlands Induced Pluripotent Stem Cells Market Future Outlook
The Netherlands induced pluripotent stem cells market is expected to witness significant growth in the coming years, driven by increasing investments in research and development, growing collaborations between academia and industry, and rising prevalence of chronic diseases. The country`s strong infrastructure for life sciences and biotechnology, coupled with supportive government initiatives, will further fuel market expansion. Additionally, the increasing adoption of regenerative medicine and personalized therapies is expected to boost demand for induced pluripotent stem cells in various applications such as drug discovery, disease modeling, and tissue engineering. Overall, the Netherlands is poised to become a key player in the global induced pluripotent stem cells market, attracting both domestic and international investments in this rapidly evolving field.
Key Highlights of the Report:
- Netherlands Induced Pluripotent Stem Cells Market Outlook
- Market Size of Netherlands Induced Pluripotent Stem Cells Market,2024
- Forecast of Netherlands Induced Pluripotent Stem Cells Market, 2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Revenues & Volume for the Period 2021-2031
- Netherlands Induced Pluripotent Stem Cells Market Trend Evolution
- Netherlands Induced Pluripotent Stem Cells Market Drivers and Challenges
- Netherlands Induced Pluripotent Stem Cells Price Trends
- Netherlands Induced Pluripotent Stem Cells Porter's Five Forces
- Netherlands Induced Pluripotent Stem Cells Industry Life Cycle
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Source Type for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Human iPSCs for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Mouse iPSCs for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Genetically Modified iPSCs for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Tissue-Specific iPSCs for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Application for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Disease Modeling for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Drug Discovery for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Stem Cell Therapy for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Personalized Medicine for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By End User for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Pharmaceutical Companies for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Academic Research Centers for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Hospitals for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Biotech Companies for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Differentiation Capability for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Multipotent for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Pluripotent for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Totipotent for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Oligopotent for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Research Stage for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Preclinical Trials for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Clinical Trials for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By Commercial Development for the Period 2021-2031
- Historical Data and Forecast of Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume By -Ready Therapies for the Period 2021 - 2029
- Netherlands Induced Pluripotent Stem Cells Import Export Trade Statistics
- Market Opportunity Assessment By Source Type
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- Market Opportunity Assessment By Differentiation Capability
- Market Opportunity Assessment By Research Stage
- Netherlands Induced Pluripotent Stem Cells Top Companies Market Share
- Netherlands Induced Pluripotent Stem Cells Competitive Benchmarking By Technical and Operational Parameters
- Netherlands Induced Pluripotent Stem Cells Company Profiles
- Netherlands Induced Pluripotent Stem Cells Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Netherlands Induced Pluripotent Stem Cells Market Overview |
3.1 Netherlands Country Macro Economic Indicators |
3.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, 2021 & 2031F |
3.3 Netherlands Induced Pluripotent Stem Cells Market - Industry Life Cycle |
3.4 Netherlands Induced Pluripotent Stem Cells Market - Porter's Five Forces |
3.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume Share, By Source Type, 2021 & 2031F |
3.6 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume Share, By Application, 2021 & 2031F |
3.7 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume Share, By End User, 2021 & 2031F |
3.8 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume Share, By Differentiation Capability, 2021 & 2031F |
3.9 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume Share, By Research Stage, 2021 & 2031F |
4 Netherlands Induced Pluripotent Stem Cells Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing research and development activities in the field of induced pluripotent stem cells in the Netherlands |
4.2.2 Rising prevalence of chronic diseases driving the demand for innovative treatment options |
4.2.3 Supportive government initiatives and funding for stem cell research and regenerative medicine |
4.3 Market Restraints |
4.3.1 Ethical concerns and regulatory restrictions surrounding the use of induced pluripotent stem cells |
4.3.2 High costs associated with research, development, and commercialization of stem cell-based therapies |
5 Netherlands Induced Pluripotent Stem Cells Market Trends |
6 Netherlands Induced Pluripotent Stem Cells Market, By Types |
6.1 Netherlands Induced Pluripotent Stem Cells Market, By Source Type |
6.1.1 Overview and Analysis |
6.1.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Source Type, 2021 - 2031F |
6.1.3 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Human iPSCs, 2021 - 2031F |
6.1.4 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Mouse iPSCs, 2021 - 2031F |
6.1.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Genetically Modified iPSCs, 2021 - 2031F |
6.1.6 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Tissue-Specific iPSCs, 2021 - 2031F |
6.2 Netherlands Induced Pluripotent Stem Cells Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Disease Modeling, 2021 - 2031F |
6.2.3 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Drug Discovery, 2021 - 2031F |
6.2.4 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Stem Cell Therapy, 2021 - 2031F |
6.2.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Personalized Medicine, 2021 - 2031F |
6.3 Netherlands Induced Pluripotent Stem Cells Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Pharmaceutical Companies, 2021 - 2031F |
6.3.3 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Academic Research Centers, 2021 - 2031F |
6.3.4 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Hospitals, 2021 - 2031F |
6.3.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Biotech Companies, 2021 - 2031F |
6.4 Netherlands Induced Pluripotent Stem Cells Market, By Differentiation Capability |
6.4.1 Overview and Analysis |
6.4.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Multipotent, 2021 - 2031F |
6.4.3 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Pluripotent, 2021 - 2031F |
6.4.4 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Totipotent, 2021 - 2031F |
6.4.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Oligopotent, 2021 - 2031F |
6.5 Netherlands Induced Pluripotent Stem Cells Market, By Research Stage |
6.5.1 Overview and Analysis |
6.5.2 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Preclinical Trials, 2021 - 2031F |
6.5.3 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Clinical Trials, 2021 - 2031F |
6.5.4 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By Commercial Development, 2021 - 2031F |
6.5.5 Netherlands Induced Pluripotent Stem Cells Market Revenues & Volume, By -Ready Therapies, 2021 - 2031F |
7 Netherlands Induced Pluripotent Stem Cells Market Import-Export Trade Statistics |
7.1 Netherlands Induced Pluripotent Stem Cells Market Export to Major Countries |
7.2 Netherlands Induced Pluripotent Stem Cells Market Imports from Major Countries |
8 Netherlands Induced Pluripotent Stem Cells Market Key Performance Indicators |
8.1 Number of research grants awarded for induced pluripotent stem cell research in the Netherlands |
8.2 Percentage of successful clinical trials using induced pluripotent stem cells |
8.3 Adoption rate of induced pluripotent stem cell therapies in clinical settings |
9 Netherlands Induced Pluripotent Stem Cells Market - Opportunity Assessment |
9.1 Netherlands Induced Pluripotent Stem Cells Market Opportunity Assessment, By Source Type, 2021 & 2031F |
9.2 Netherlands Induced Pluripotent Stem Cells Market Opportunity Assessment, By Application, 2021 & 2031F |
9.3 Netherlands Induced Pluripotent Stem Cells Market Opportunity Assessment, By End User, 2021 & 2031F |
9.4 Netherlands Induced Pluripotent Stem Cells Market Opportunity Assessment, By Differentiation Capability, 2021 & 2031F |
9.5 Netherlands Induced Pluripotent Stem Cells Market Opportunity Assessment, By Research Stage, 2021 & 2031F |
10 Netherlands Induced Pluripotent Stem Cells Market - Competitive Landscape |
10.1 Netherlands Induced Pluripotent Stem Cells Market Revenue Share, By Companies, 2024 |
10.2 Netherlands Induced Pluripotent Stem Cells Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















