Chile Suture Needles Market Outlook | Trends, Size, Analysis, Share, Value, Companies, Revenue, COVID-19 IMPACT, Forecast, Industry & Growth
| Product Code: ETC369788 | Publication Date: Aug 2022 | Updated Date: Jul 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Summon Dutta | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Chile Suture Needles Market Size Growth Rate
The Chile Suture Needles Market is projected to witness mixed growth rate patterns during 2025 to 2029. Growth accelerates to 5.26% in 2027, following an initial rate of 4.87%, before easing to 3.61% at the end of the period.
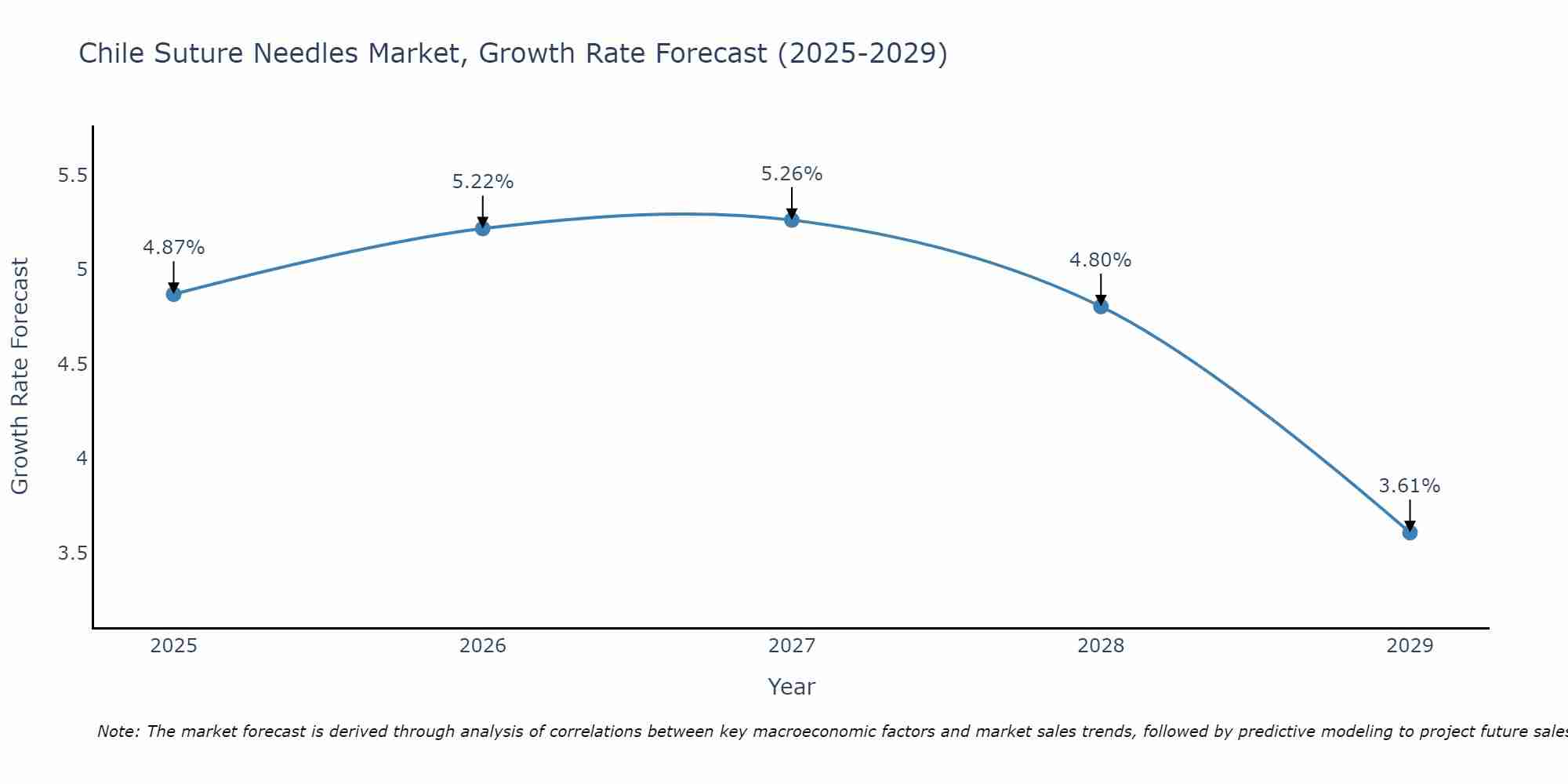
Chile Suture Needles Market Synopsis
The Chile Suture Needles market is experiencing steady growth due to increasing surgical procedures, rising geriatric population, and advancements in healthcare infrastructure. The market is primarily driven by the demand for surgical sutures in various medical procedures, such as cardiovascular surgeries, orthopedic surgeries, and cosmetic surgeries. Key players in the market are focusing on product innovations, such as the development of absorbable sutures and needle technology enhancements, to meet the evolving requirements of healthcare professionals. Additionally, the growing awareness about the importance of wound care and the increasing prevalence of chronic diseases are also contributing to the market growth. The market is anticipated to witness further expansion with the adoption of minimally invasive surgical techniques and the increasing investments in healthcare facilities.
Chile Suture Needles Market Trends
The Chile Suture Needles Market is witnessing several key trends. One significant trend is the increasing adoption of advanced suture needle materials such as stainless steel, titanium, and polymer-coated needles, which offer enhanced strength, durability, and reduced tissue trauma. Another trend is the growing preference for minimally invasive procedures, driving the demand for smaller, finer suture needles that allow for precise and efficient suturing in delicate surgeries. Moreover, there is a rising focus on developing suturing techniques that promote faster wound healing and minimize scarring, leading to the introduction of innovative suture needle designs and configurations. Additionally, the market is experiencing a shift towards the use of disposable and safety-engineered suture needles to reduce the risk of needlestick injuries and infections, in line with stringent healthcare regulations and standards.
Chile Suture Needles Market Challenges
In the Chile suture needles market, several challenges are faced by manufacturers and healthcare providers. One of the key challenges is the increasing competition from both domestic and international players, leading to pricing pressures and the need for continuous innovation to stay competitive. Another challenge is the stringent regulatory requirements for the approval and sale of suture needles, which can create barriers to entry for new companies and hinder market growth. Additionally, the market faces challenges related to the availability of skilled healthcare professionals who are proficient in using advanced suture needle technologies, as well as the need for efficient distribution channels to ensure timely access to these products across the country. Overall, navigating these challenges requires strategic planning and a deep understanding of the evolving market dynamics in Chile.
Chile Suture Needles Market Investment Opportunities
The Chile suture needles market presents promising investment opportunities due to factors such as increasing surgical procedures, growing healthcare infrastructure, and a rising geriatric population. With advancements in technology leading to the development of innovative suture needles that offer better precision and reduced tissue trauma, there is a growing demand for these products in the healthcare sector. Investing in companies that manufacture suture needles or provide related services in Chile could prove to be lucrative as the market continues to expand. Additionally, partnerships with healthcare facilities and strategic distribution channels can further enhance growth prospects in this sector. Overall, the Chile suture needles market offers potential for investors looking to capitalize on the increasing demand for medical devices in the country.
Jordan Agar Market Government Policies
The Chilean government has implemented regulations to ensure the safety and quality of suture needles in the market. The Ministry of Health oversees the registration and approval process for medical devices, including suture needles, to guarantee compliance with national standards and requirements. Additionally, importation and distribution of suture needles are subject to strict regulations to safeguard public health and prevent the entry of substandard products into the market. The government also works to promote innovation and technological advancements in the healthcare sector, encouraging the development of new and improved suture needle products that meet international standards. Overall, government policies in Chile aim to regulate and monitor the suture needles market to ensure patient safety and product quality.
Chile Suture Needles Market Future Outlook
The Chile suture needles market is expected to show steady growth in the coming years, driven by factors such as increasing surgical procedures, advancements in healthcare infrastructure, and a growing geriatric population. The rise in chronic diseases and the expanding healthcare sector in Chile are also contributing to the demand for suture needles. Technological advancements in suture materials and designs are further expected to fuel market growth. Additionally, the focus on minimizing post-operative complications and improving patient outcomes will drive the adoption of innovative suture needles in the country. However, factors such as stringent regulatory requirements and the availability of alternative wound closure methods may pose challenges to market growth. Overall, the Chile suture needles market is projected to experience moderate yet sustainable growth in the foreseeable future.
Key Highlights of the Report:
- Chile Suture Needles Market Outlook
- Market Size of Chile Suture Needles Market, 2021
- Forecast of Chile Suture Needles Market, 2031
- Historical Data and Forecast of Chile Suture Needles Revenues & Volume for the Period 2018 - 2031
- Chile Suture Needles Market Trend Evolution
- Chile Suture Needles Market Drivers and Challenges
- Chile Suture Needles Price Trends
- Chile Suture Needles Porter's Five Forces
- Chile Suture Needles Industry Life Cycle
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Type for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Round Bodied Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Blunt Point Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Reverse Cutting Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Conventional Cutting Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Spatula Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Tapercut Needle for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Application for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Hospital for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Clinics for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Ambulatory Surgical Centres for the Period 2018 - 2031
- Historical Data and Forecast of Chile Suture Needles Market Revenues & Volume By Others for the Period 2018 - 2031
- Chile Suture Needles Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Application
- Chile Suture Needles Top Companies Market Share
- Chile Suture Needles Competitive Benchmarking By Technical and Operational Parameters
- Chile Suture Needles Company Profiles
- Chile Suture Needles Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















