China PAP and Paracetamol Market (2025-2031) | Industry, Growth, Competitive Landscape, Forecast, Trends, Outlook, Size & Revenue, Value, Segmentation, Companies, Share, Analysis
| Product Code: ETC6749750 | Publication Date: Sep 2024 | Updated Date: Oct 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
China Pap and Paracetamol Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, China continued to rely on imports for pap and paracetamol, with top exporters being Puerto Rico, Metropolitan France, Germany, Spain, and Italy. Despite a high concentration with a high Herfindahl-Hirschman Index (HHI) in 2024, the compound annual growth rate (CAGR) from 2020 to 2024 was -2.06%, indicating a decline in overall import demand. The growth rate from 2023 to 2024 saw a significant decrease of -26.63%, highlighting potential challenges in the market for these pharmaceutical products in China.
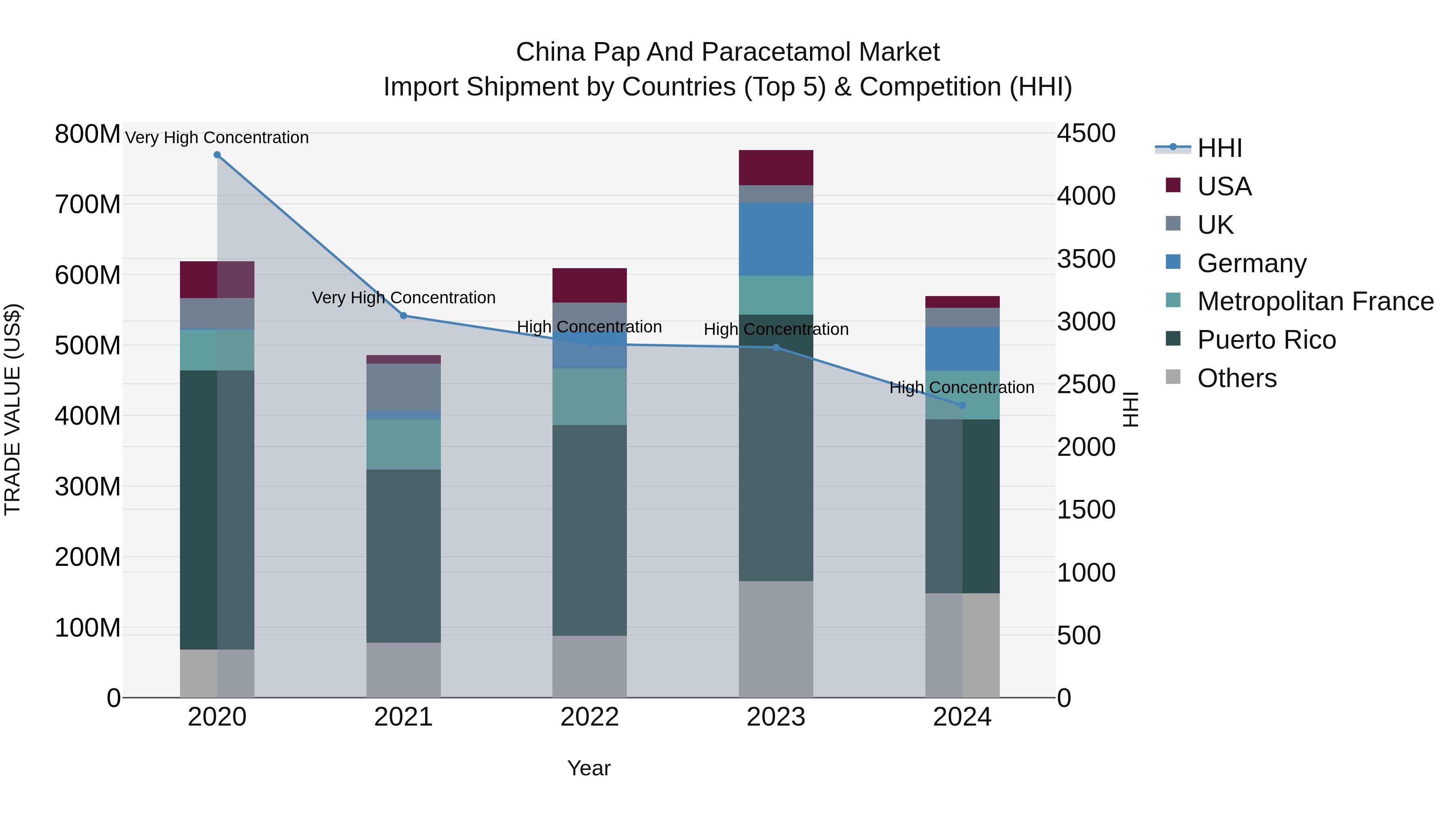
China PAP and Paracetamol Market Synopsis
The China PAP (Para-Amino Phenol) and Paracetamol market is witnessing significant growth driven by factors such as increasing healthcare expenditure, a growing aging population, and rising prevalence of chronic diseases. PAP is a key raw material used in the production of Paracetamol, a widely used pain reliever and fever reducer medication. China is one of the largest producers and exporters of Paracetamol globally, with a number of key players in the market. The market is characterized by intense competition, technological advancements, and increasing investments in research and development to enhance product offerings and expand market reach. With a growing emphasis on healthcare infrastructure and pharmaceutical development in China, the PAP and Paracetamol market is expected to continue its growth trajectory in the coming years.
China PAP and Paracetamol Market Trends
The China PAP (Para-Amino-Phenol) and Paracetamol market is experiencing steady growth due to increasing demand for pain relief medications and the expanding pharmaceutical industry in the country. The growing prevalence of chronic diseases and rising healthcare expenditure are driving the market`s growth. Additionally, the emphasis on quality healthcare and the government`s initiatives to improve access to essential medications are creating opportunities for manufacturers and suppliers in the market. With the increasing focus on research and development, innovation in drug formulations, and strategic partnerships, the China PAP and Paracetamol market is expected to witness further expansion and advancements in the coming years. Companies operating in this market have the potential to capitalize on these trends by offering high-quality products and expanding their market presence through effective marketing strategies and distribution channels.
China PAP and Paracetamol Market Challenges
In the China PAP (Para-Amino-Phenol) and Paracetamol market, some key challenges include regulatory issues related to quality control and adherence to safety standards, intense competition among manufacturers leading to price wars and margin pressures, counterfeiting and product adulteration concerns impacting consumer trust, and the need for continuous innovation to meet evolving customer preferences and regulatory requirements. Additionally, fluctuations in raw material prices, supply chain disruptions, and changing government regulations can also pose challenges for companies operating in this market. Navigating these complexities requires a strategic approach that focuses on quality assurance, compliance with regulations, brand protection, and value-added product differentiation to maintain a competitive edge and sustain growth in the China PAP and Paracetamol market.
China PAP and Paracetamol Market Investment Opportunities
The China PAP (Para-Amino-Phenol) and Paracetamol market are primarily driven by factors such as increasing healthcare expenditures, rising prevalence of chronic diseases, growing aging population, and the expanding pharmaceutical industry in the country. Additionally, the demand for pain relief medications like Paracetamol is rising due to lifestyle changes, urbanization, and a higher incidence of conditions such as headaches, fever, and musculoskeletal disorders. The government`s initiatives to improve healthcare infrastructure and access to essential medications also play a significant role in driving the market growth. Furthermore, the increasing consumer awareness regarding self-medication and over-the-counter drugs further propels the demand for Paracetamol and PAP products in China.
China PAP and Paracetamol Market Government Polices
The Chinese government has implemented various policies related to the Paracetamol Active Pharmaceutical Ingredient (PAP) and Paracetamol Market, aiming to regulate the production, quality, and export of these pharmaceutical products. These policies include stringent quality control measures to ensure the safety and efficacy of Paracetamol products, as well as regulations on production standards and environmental protection in PAP manufacturing facilities. Additionally, the government has imposed export restrictions on Paracetamol and PAP to prevent overcapacity and maintain market stability. These policies are crucial in safeguarding public health, promoting sustainable production practices, and supporting the growth of the Chinese pharmaceutical industry while ensuring compliance with international standards and trade regulations.
China PAP and Paracetamol Market Future Outlook
The future outlook for the China Paracetamol (PAP) and paracetamol market appears promising due to several factors. The increasing prevalence of chronic diseases, rising geriatric population, and growing consumer awareness about healthcare are expected to drive the demand for paracetamol-based products in China. Additionally, the government`s initiatives to expand healthcare access and improve pharmaceutical regulations are likely to support market growth. With the ongoing advancements in pharmaceutical manufacturing technologies and increasing investments in research and development, there is a potential for innovation and introduction of new paracetamol formulations in the market. However, the market may face challenges such as pricing pressures and competition from generic products. Overall, the China PAP and paracetamol market are anticipated to witness steady growth in the coming years.
Key Highlights of the Report:
- China PAP and Paracetamol Market Outlook
- Market Size of China PAP and Paracetamol Market, 2024
- Forecast of China PAP and Paracetamol Market, 2031
- Historical Data and Forecast of China PAP and Paracetamol Revenues & Volume for the Period 2021- 2031
- China PAP and Paracetamol Market Trend Evolution
- China PAP and Paracetamol Market Drivers and Challenges
- China PAP and Paracetamol Price Trends
- China PAP and Paracetamol Porter's Five Forces
- China PAP and Paracetamol Industry Life Cycle
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Dosage Form for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Powder for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Granules for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Tablet Drug for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Granules Drug for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Oral Solutions for the Period 2021- 2031
- Historical Data and Forecast of China PAP and Paracetamol Market Revenues & Volume By Other Applications for the Period 2021- 2031
- China PAP and Paracetamol Import Export Trade Statistics
- Market Opportunity Assessment By Dosage Form
- Market Opportunity Assessment By Application
- China PAP and Paracetamol Top Companies Market Share
- China PAP and Paracetamol Competitive Benchmarking By Technical and Operational Parameters
- China PAP and Paracetamol Company Profiles
- China PAP and Paracetamol Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 China PAP and Paracetamol Market Overview |
3.1 China Country Macro Economic Indicators |
3.2 China PAP and Paracetamol Market Revenues & Volume, 2021 & 2031F |
3.3 China PAP and Paracetamol Market - Industry Life Cycle |
3.4 China PAP and Paracetamol Market - Porter's Five Forces |
3.5 China PAP and Paracetamol Market Revenues & Volume Share, By Dosage Form, 2021 & 2031F |
3.6 China PAP and Paracetamol Market Revenues & Volume Share, By Application, 2021 & 2031F |
4 China PAP and Paracetamol Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing awareness about the benefits of using pap and paracetamol in healthcare. |
4.2.2 Growing elderly population in China leading to higher demand for pain relief medications. |
4.2.3 Rising incidence of chronic diseases and infections driving the need for pap and paracetamol consumption. |
4.3 Market Restraints |
4.3.1 Stringent regulations and approvals required for the manufacturing and distribution of pap and paracetamol products. |
4.3.2 Presence of counterfeit and substandard products impacting the credibility of the market. |
4.3.3 Price fluctuations in raw materials affecting the production costs of pap and paracetamol. |
5 China PAP and Paracetamol Market Trends |
6 China PAP and Paracetamol Market, By Types |
6.1 China PAP and Paracetamol Market, By Dosage Form |
6.1.1 Overview and Analysis |
6.1.2 China PAP and Paracetamol Market Revenues & Volume, By Dosage Form, 2021- 2031F |
6.1.3 China PAP and Paracetamol Market Revenues & Volume, By Powder, 2021- 2031F |
6.1.4 China PAP and Paracetamol Market Revenues & Volume, By Granules, 2021- 2031F |
6.2 China PAP and Paracetamol Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 China PAP and Paracetamol Market Revenues & Volume, By Tablet Drug, 2021- 2031F |
6.2.3 China PAP and Paracetamol Market Revenues & Volume, By Granules Drug, 2021- 2031F |
6.2.4 China PAP and Paracetamol Market Revenues & Volume, By Oral Solutions, 2021- 2031F |
6.2.5 China PAP and Paracetamol Market Revenues & Volume, By Other Applications, 2021- 2031F |
7 China PAP and Paracetamol Market Import-Export Trade Statistics |
7.1 China PAP and Paracetamol Market Export to Major Countries |
7.2 China PAP and Paracetamol Market Imports from Major Countries |
8 China PAP and Paracetamol Market Key Performance Indicators |
8.1 Number of new product launches in the pap and paracetamol market. |
8.2 Percentage of market share held by key players in the industry. |
8.3 Consumer satisfaction levels with pap and paracetamol products. |
8.4 Rate of adoption of pap and paracetamol in different healthcare settings. |
8.5 Number of clinical trials and research studies conducted on the efficacy of pap and paracetamol. |
9 China PAP and Paracetamol Market - Opportunity Assessment |
9.1 China PAP and Paracetamol Market Opportunity Assessment, By Dosage Form, 2021 & 2031F |
9.2 China PAP and Paracetamol Market Opportunity Assessment, By Application, 2021 & 2031F |
10 China PAP and Paracetamol Market - Competitive Landscape |
10.1 China PAP and Paracetamol Market Revenue Share, By Companies, 2024 |
10.2 China PAP and Paracetamol Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















