Colombia Bone Grafts And Substitutes Market (2025-2031) Outlook | Analysis, Forecast, Share, Companies, Industry, Value, Size, Revenue, Trends & Growth
| Product Code: ETC380464 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Colombia Bone Grafts and Substitutes Market: Top 5 Importing Countries and Market Competition (HHI) Analysis
The Colombia bone grafts and substitutes import market experienced significant growth in 2024, with the United States, Germany, Italy, France, and Argentina emerging as the top exporting countries. The market concentration, as measured by the HHI, shifted from moderate to high in 2024, indicating increased dominance by key players. The impressive compound annual growth rate (CAGR) of 22.84% from 2020 to 2024 demonstrates a thriving market, while the 21.16% growth rate from 2023 to 2024 underscores the continued momentum and opportunities in this sector.
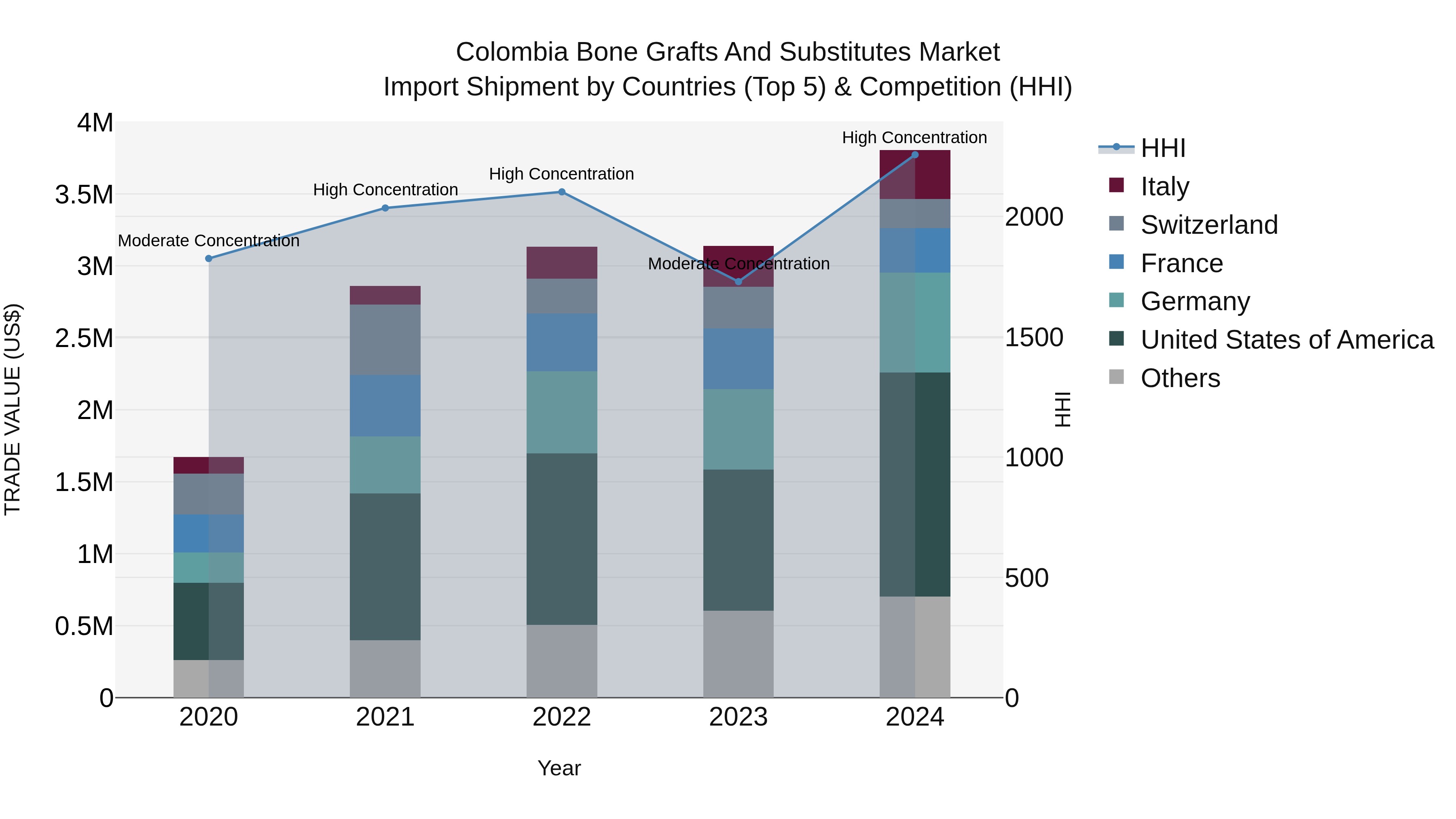
Colombia Bone Grafts And Substitutes Market Synopsis
The Colombia Bone Grafts and Substitutes market is experiencing steady growth due to increasing incidences of bone disorders and orthopedic surgeries. The market is driven by factors such as a growing aging population, rising awareness about the benefits of bone grafts in medical procedures, and advancements in healthcare infrastructure. The demand for bone grafts and substitutes is also being fueled by the rising prevalence of osteoporosis and other bone-related diseases. Key players in the market are focusing on product innovations, strategic collaborations, and mergers to gain a competitive edge. Additionally, the adoption of technologically advanced products like synthetic bone grafts and stem cell-based therapies is expected to further drive market growth in Colombia.
Colombia Bone Grafts And Substitutes Market Trends
The Colombia Bone Grafts and Substitutes market is witnessing a shift towards innovative products that offer enhanced biocompatibility and faster healing times. There is a growing demand for synthetic bone graft substitutes due to their consistent quality and reduced risk of disease transmission compared to traditional allografts. Additionally, the market is seeing a rise in the adoption of regenerative medicine approaches, such as stem cell therapy and growth factor-based products, to stimulate bone regeneration. With an increasing emphasis on minimally invasive procedures and the rising prevalence of orthopedic conditions, the Colombia Bone Grafts and Substitutes market is expected to continue its growth trajectory, driven by advancements in material science and biotechnology.
Colombia Bone Grafts And Substitutes Market Challenges
One major challenge faced in the Colombia Bone Grafts and Substitutes Market is the limited awareness and adoption of advanced bone grafting technologies among healthcare providers and patients. The market is dominated by traditional bone grafting methods, such as autografts and allografts, due to familiarity and perceived reliability. This poses a barrier to the uptake of newer, more innovative bone graft substitutes that offer benefits such as reduced infection risk and faster healing times. Additionally, regulatory hurdles and reimbursement limitations can hinder market growth as companies face challenges in gaining approval and coverage for their products. Overcoming these challenges will require targeted education efforts, collaboration between industry stakeholders and healthcare professionals, and advancements in regulatory processes to facilitate the adoption of advanced bone graft technologies in Colombia.
Colombia Bone Grafts And Substitutes Market Investment Opportunities
The Colombia Bone Grafts and Substitutes Market presents promising investment opportunities due to a growing demand for orthopedic procedures and an increasing prevalence of bone-related disorders in the country. The market is driven by factors such as the rising geriatric population, expanding healthcare infrastructure, and advancements in medical technology. Companies offering innovative bone graft products, such as synthetic bone substitutes and demineralized bone matrix, are well-positioned to capitalize on this market growth. Additionally, strategic collaborations with healthcare providers, research institutions, and regulatory bodies can help investors navigate the market landscape and establish a strong presence in Colombia`s healthcare sector. Overall, the Colombia Bone Grafts and Substitutes Market offers potential for sustainable growth and attractive returns on investment for stakeholders.
Jordan Agar Market Government Policies
In Colombia, the bone grafts and substitutes market is regulated by the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA), which oversees the registration, importation, and commercialization of medical products, including bone grafts. Companies seeking to enter the market must comply with INVIMA`s requirements for product safety, quality, and efficacy through the submission of registration dossiers and adherence to Good Manufacturing Practices (GMP). Additionally, the Colombian government has implemented policies to promote the development and adoption of innovative medical technologies, including bone graft substitutes, through tax incentives, research grants, and partnerships with academic institutions. Overall, the regulatory framework aims to ensure patient safety, foster competition, and encourage advancements in the bone grafts and substitutes market in Colombia.
Colombia Bone Grafts And Substitutes Market Future Outlook
The Colombia Bone Grafts and Substitutes Market is expected to witness steady growth in the coming years due to the increasing prevalence of orthopedic and dental conditions, coupled with advancements in healthcare infrastructure. The market is also likely to benefit from rising awareness about the importance of bone grafts in promoting bone healing and regeneration. Additionally, the growing geriatric population in Colombia is anticipated to drive the demand for bone grafts and substitutes as age-related bone disorders become more prevalent. Technological innovations in graft materials and surgical techniques are further expected to fuel market growth. However, regulatory challenges and pricing pressures may pose some hindrances to market expansion. Overall, the Colombia Bone Grafts and Substitutes Market is poised for gradual growth, supported by evolving healthcare trends and increasing patient preference for minimally invasive procedures.
Key Highlights of the Report:
- Colombia Bone Grafts And Substitutes Market Outlook
- Market Size of Colombia Bone Grafts And Substitutes Market, 2024
- Forecast of Colombia Bone Grafts And Substitutes Market, 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Revenues & Volume for the Period 2021 - 2031
- Colombia Bone Grafts And Substitutes Market Trend Evolution
- Colombia Bone Grafts And Substitutes Market Drivers and Challenges
- Colombia Bone Grafts And Substitutes Price Trends
- Colombia Bone Grafts And Substitutes Porter's Five Forces
- Colombia Bone Grafts And Substitutes Industry Life Cycle
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Material Type for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Allograft for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Synthetic for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Composites for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Polymers for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Bone Morphogenic Proteins (BMP) for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Craniomaxillofacial for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Dental for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Foot & Ankle for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Joint Reconstruction for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Long Bone for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Bone Grafts And Substitutes Market Revenues & Volume By Spinal Fusion for the Period 2021 - 2031
- Colombia Bone Grafts And Substitutes Import Export Trade Statistics
- Market Opportunity Assessment By Material Type
- Market Opportunity Assessment By Application
- Colombia Bone Grafts And Substitutes Top Companies Market Share
- Colombia Bone Grafts And Substitutes Competitive Benchmarking By Technical and Operational Parameters
- Colombia Bone Grafts And Substitutes Company Profiles
- Colombia Bone Grafts And Substitutes Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















