France Anesthesia Devices Market (2025-2031) Outlook | Value, Forecast, Trends, Size, Share, Industry, Revenue, Companies, Growth & Analysis
| Product Code: ETC361750 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
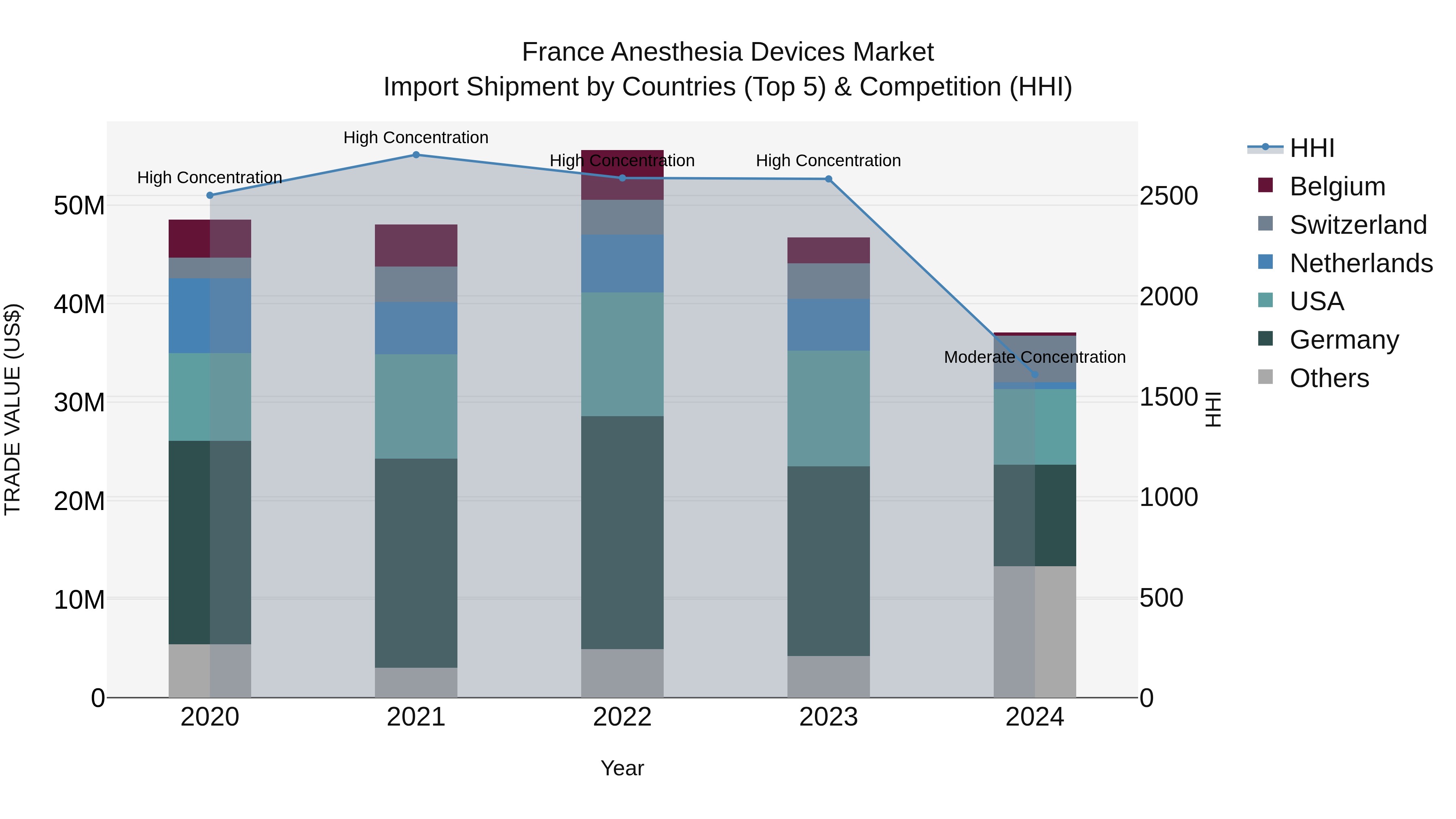
France Anesthesia Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s anesthesia devices import market in 2024 saw a shift in concentration levels from high to moderate, with a notable decrease in the CAGR from 2020 to 2024 at -6.52%. The top exporting countries to France included Germany, USA, Switzerland, Pakistan, and China. Despite the overall negative growth rate of -20.67% from 2023 to 2024, the market dynamics suggest opportunities for diversification and potential for new market entrants to capitalize on the evolving landscape.
France Anesthesia Devices Market Synopsis
The France Anesthesia Devices Market is a mature and well-established market, driven by factors such as the increasing number of surgeries, advancements in technology, and a growing geriatric population. Key players in the market are focusing on product innovation, such as the development of advanced anesthesia machines and monitoring devices. The market is highly competitive, with companies adopting strategies like mergers and acquisitions to strengthen their market presence. With a strong healthcare infrastructure and high adoption of advanced medical technologies, France presents significant opportunities for growth in the anesthesia devices market. However, regulatory challenges and pricing pressures pose as key restraints for market expansion. Overall, the France Anesthesia Devices Market is poised for steady growth, supported by increasing healthcare expenditure and a rising demand for minimally invasive procedures.
France Anesthesia Devices Market Trends
The France Anesthesia Devices Market is witnessing several notable trends. One key trend is the increasing adoption of advanced anesthesia equipment and technologies, such as anesthesia monitors with enhanced features for patient safety and precise drug delivery systems. Another trend is the growing demand for portable and compact anesthesia devices for use in ambulatory surgery centers and remote healthcare settings. Additionally, there is a rising focus on the development of anesthesia devices with integrated data management and connectivity capabilities to streamline workflow and improve efficiency in healthcare facilities. Moreover, the market is experiencing a shift towards the use of disposable anesthesia devices to reduce the risk of infections and cross-contamination. Overall, these trends indicate a move towards more sophisticated, portable, and interconnected anesthesia devices in the France market.
France Anesthesia Devices Market Challenges
In the France Anesthesia Devices Market, some of the key challenges faced include stringent regulatory requirements, increasing competition among market players, and the need for advanced technology and innovation to meet the evolving demands of healthcare professionals. The regulatory landscape in France is complex, requiring rigorous adherence to standards and guidelines, which can pose challenges for manufacturers in terms of compliance and time-to-market. Additionally, the market is highly competitive with a growing number of players offering similar products, leading to pricing pressures and the need for differentiation. Moreover, as healthcare providers seek more efficient and reliable anesthesia solutions, there is a constant demand for innovative technologies that improve patient outcomes and enhance overall safety in anesthesia delivery. Meeting these challenges requires continuous investment in research and development to stay ahead in the competitive landscape of the France Anesthesia Devices Market.
France Anesthesia Devices Market Investment Opportunities
The France Anesthesia Devices Market offers various investment opportunities due to the growing demand for advanced medical equipment in the healthcare sector. Key areas for investment include innovative anesthesia delivery systems, patient monitoring devices, and anesthesia masks. With the increasing prevalence of chronic diseases and the aging population in France, there is a rising need for efficient anesthesia devices to improve patient care and surgical outcomes. Additionally, advancements in technology such as the integration of artificial intelligence and data analytics in anesthesia devices present opportunities for investors to capitalize on the market. Investing in companies that focus on developing cutting-edge anesthesia devices and expanding their market presence in France can be a strategic move to benefit from the market growth and potential profitability in the long term.
Jordan Agar Market Government Policies
The France Anesthesia Devices Market is regulated by various government policies aimed at ensuring patient safety and quality of care. The French regulatory authority, Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM), oversees the approval and monitoring of anesthesia devices to ensure they meet safety and efficacy standards. Additionally, the European Medical Devices Regulation (MDR) sets out requirements for the design, manufacture, and marketing of medical devices, including anesthesia devices, to enhance patient safety and ensure product quality. These regulations also aim to promote innovation and competitiveness in the market while safeguarding public health. Compliance with these policies is essential for manufacturers and healthcare providers operating in the France Anesthesia Devices Market to ensure the delivery of safe and effective anesthesia care.
France Anesthesia Devices Market Future Outlook
The France Anesthesia Devices Market is expected to witness steady growth in the coming years due to the increasing demand for advanced anesthesia equipment in healthcare facilities. Factors such as a growing geriatric population, rising surgical procedures, and technological advancements in anesthesia devices are driving market expansion. The market is also benefiting from the increasing focus on patient safety and the adoption of minimally invasive procedures. However, regulatory challenges and the high cost of anesthesia devices may pose some restraints. Overall, with the ongoing advancements in technology and increasing healthcare investments, the France Anesthesia Devices Market is anticipated to experience sustained growth and innovation in the foreseeable future.
Key Highlights of the Report:
- France Anesthesia Devices Market Outlook
- Market Size of France Anesthesia Devices Market, 2024
- Forecast of France Anesthesia Devices Market, 2031
- Historical Data and Forecast of France Anesthesia Devices Revenues & Volume for the Period 2021 - 2031
- France Anesthesia Devices Market Trend Evolution
- France Anesthesia Devices Market Drivers and Challenges
- France Anesthesia Devices Price Trends
- France Anesthesia Devices Porter's Five Forces
- France Anesthesia Devices Industry Life Cycle
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Product for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Anesthesia Delivery Machine for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Anesthesia Disposables & Accessories for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Anesthesia Monitors for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Anesthesia Information Management Systems for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Cardiology for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Neurology for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Dental for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Ophthalmology for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Urology for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Orthopedics for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By End User for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Hospital for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Clinics for the Period 2021 - 2031
- Historical Data and Forecast of France Anesthesia Devices Market Revenues & Volume By Ambulatory Service Centres for the Period 2021 - 2031
- France Anesthesia Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- France Anesthesia Devices Top Companies Market Share
- France Anesthesia Devices Competitive Benchmarking By Technical and Operational Parameters
- France Anesthesia Devices Company Profiles
- France Anesthesia Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















