France Diagnostic Reagent Market Outlook | Trends, Growth, Analysis, Forecast, Size, Value, Companies, Industry, Revenue, COVID-19 IMPACT & Share
| Product Code: ETC419106 | Publication Date: Oct 2022 | Updated Date: Aug 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
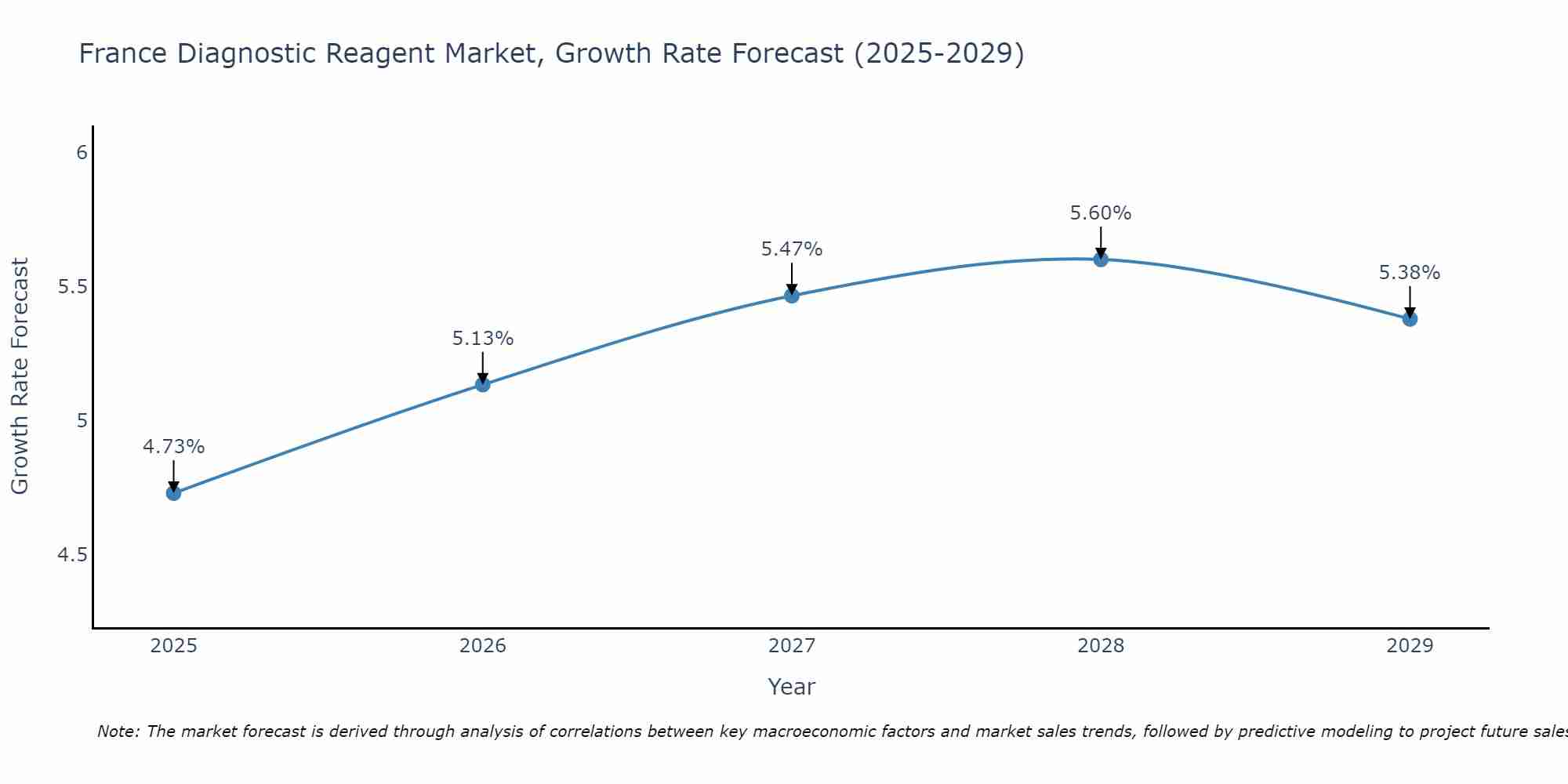
France Diagnostic Reagent Market Size Growth Rate
The France Diagnostic Reagent Market is projected to witness mixed growth rate patterns during 2025 to 2029. Growth accelerates to 5.60% in 2028, following an initial rate of 4.73%, before easing to 5.38% at the end of the period.
France Diagnostic Reagent Market Synopsis
The France Diagnostic Reagent Market is a dynamic and expanding sector characterized by a wide range of products catering to various medical testing needs. Key players in the market offer diagnostic reagents for applications such as clinical chemistry, immunochemistry, microbiology, and molecular diagnostics. The market is driven by factors such as increasing prevalence of chronic and infectious diseases, advancements in diagnostic technologies, and a growing emphasis on early disease detection. Additionally, the rising demand for personalized medicine and point-of-care testing solutions further fuels market growth. Competition among market players is intense, leading to continuous innovation and product development to meet evolving customer requirements. Overall, the France Diagnostic Reagent Market presents significant opportunities for growth and investment in the coming years.
France Diagnostic Reagent Market Trends
The France Diagnostic Reagent Market is experiencing several key trends. One prominent trend is the increasing demand for innovative diagnostic solutions, driven by advancements in technology and rising prevalence of chronic diseases. There is a growing focus on personalized medicine, leading to the development of targeted diagnostic reagents. Additionally, the emphasis on early disease detection and prevention is fueling the demand for rapid and accurate diagnostic tests. Market players are also investing in research and development to introduce new and improved diagnostic reagents that offer enhanced specificity and sensitivity. Furthermore, collaborations between diagnostic reagent manufacturers and healthcare providers are on the rise to streamline diagnostic processes and improve patient outcomes. Overall, the France Diagnostic Reagent Market is witnessing a shift towards more precise, efficient, and personalized diagnostic solutions.
France Diagnostic Reagent Market Challenges
In the France Diagnostic Reagent Market, challenges include increasing regulatory requirements, competition from local and international players, and the need for continuous innovation to meet evolving customer demands. Regulatory hurdles such as stringent approval processes can delay product launches and impact market entry. Local and international competition puts pressure on pricing and market share, requiring companies to differentiate themselves through quality, service, or technology. Additionally, the rapid advancements in diagnostic technologies necessitate constant innovation to stay competitive and relevant in the market. Adapting to these challenges requires companies to invest in research and development, regulatory compliance, and strategic partnerships to navigate the complexities of the France Diagnostic Reagent Market effectively.
France Diagnostic Reagent Market Investment Opportunities
The France Diagnostic Reagent Market offers promising investment opportunities due to the increasing demand for diagnostic tests in the healthcare sector. With a growing emphasis on early disease detection and personalized medicine, there is a rising need for innovative diagnostic reagents that can provide accurate and timely results. Investing in companies that specialize in developing and manufacturing diagnostic reagents for various applications such as infectious diseases, oncology, and genetic testing could prove to be lucrative. Additionally, advancements in technology, such as point-of-care testing and molecular diagnostics, are driving market growth and creating opportunities for investors to capitalize on the evolving landscape of the diagnostic reagent market in France.
Jordan Agar Market Government Policies
In France, the Diagnostic Reagent Market is subject to regulations and policies set by various government bodies. The French National Agency for Medicines and Health Products Safety (ANSM) oversees the approval and monitoring of diagnostic reagents to ensure their safety and efficacy. Additionally, the French Ministry of Health sets guidelines for the distribution and use of diagnostic reagents to protect public health. These regulations aim to maintain high standards of quality and safety in the diagnostic reagent market, ensuring that healthcare providers and patients have access to reliable and accurate diagnostic products. Compliance with these government policies is crucial for companies operating in the France Diagnostic Reagent Market to navigate the regulatory landscape and maintain market authorization for their products.
France Diagnostic Reagent Market Future Outlook
The France Diagnostic Reagent Market is poised for significant growth in the coming years due to the increasing prevalence of chronic diseases, expanding geriatric population, and rising demand for early disease detection. Technological advancements in diagnostics and the growing emphasis on personalized medicine are driving the adoption of diagnostic reagents in France. Additionally, the government`s focus on improving healthcare infrastructure and the increasing investments in research and development activities are expected to further propel market growth. The COVID-19 pandemic has also highlighted the importance of diagnostic testing, leading to a surge in demand for diagnostic reagents. Overall, the France Diagnostic Reagent Market is anticipated to experience robust expansion in the foreseeable future, presenting lucrative opportunities for market players.
Key Highlights of the Report:
- France Diagnostic Reagent Market Outlook
- Market Size of France Diagnostic Reagent Market, 2021
- Forecast of France Diagnostic Reagent Market, 2031
- Historical Data and Forecast of France Diagnostic Reagent Revenues & Volume for the Period 2018 - 2031
- France Diagnostic Reagent Market Trend Evolution
- France Diagnostic Reagent Market Drivers and Challenges
- France Diagnostic Reagent Price Trends
- France Diagnostic Reagent Porter's Five Forces
- France Diagnostic Reagent Industry Life Cycle
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Type for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Clinical Reagents for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Analytical Reagents for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Others for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Application for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Hospitals for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Laboratories for the Period 2018 - 2031
- Historical Data and Forecast of France Diagnostic Reagent Market Revenues & Volume By Research Institutions for the Period 2018 - 2031
- France Diagnostic Reagent Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Application
- France Diagnostic Reagent Top Companies Market Share
- France Diagnostic Reagent Competitive Benchmarking By Technical and Operational Parameters
- France Diagnostic Reagent Company Profiles
- France Diagnostic Reagent Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Diagnostic Reagent Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Diagnostic Reagent Market Revenues & Volume, 2021 & 2031F |
3.3 France Diagnostic Reagent Market - Industry Life Cycle |
3.4 France Diagnostic Reagent Market - Porter's Five Forces |
3.5 France Diagnostic Reagent Market Revenues & Volume Share, By Type, 2021 & 2031F |
3.6 France Diagnostic Reagent Market Revenues & Volume Share, By Application, 2021 & 2031F |
4 France Diagnostic Reagent Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of chronic diseases in France leading to higher demand for diagnostic reagents. |
4.2.2 Technological advancements in the field of diagnostics driving the adoption of innovative diagnostic reagents. |
4.2.3 Growing focus on personalized medicine leading to increased use of specialized diagnostic reagents. |
4.3 Market Restraints |
4.3.1 Stringent regulations in France governing the approval and commercialization of diagnostic reagents. |
4.3.2 High competition among market players leading to pricing pressures. |
4.3.3 Limited reimbursement policies for diagnostic reagents affecting market penetration. |
5 France Diagnostic Reagent Market Trends |
6 France Diagnostic Reagent Market, By Types |
6.1 France Diagnostic Reagent Market, By Type |
6.1.1 Overview and Analysis |
6.1.2 France Diagnostic Reagent Market Revenues & Volume, By Type, 2021-2031F |
6.1.3 France Diagnostic Reagent Market Revenues & Volume, By Clinical Reagents, 2021-2031F |
6.1.4 France Diagnostic Reagent Market Revenues & Volume, By Analytical Reagents, 2021-2031F |
6.1.5 France Diagnostic Reagent Market Revenues & Volume, By Others, 2021-2031F |
6.2 France Diagnostic Reagent Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 France Diagnostic Reagent Market Revenues & Volume, By Hospitals, 2021-2031F |
6.2.3 France Diagnostic Reagent Market Revenues & Volume, By Laboratories, 2021-2031F |
6.2.4 France Diagnostic Reagent Market Revenues & Volume, By Research Institutions, 2021-2031F |
7 France Diagnostic Reagent Market Import-Export Trade Statistics |
7.1 France Diagnostic Reagent Market Export to Major Countries |
7.2 France Diagnostic Reagent Market Imports from Major Countries |
8 France Diagnostic Reagent Market Key Performance Indicators |
8.1 Research and development investment in new diagnostic reagents. |
8.2 Adoption rate of advanced diagnostic technologies in the market. |
8.3 Number of partnerships and collaborations between diagnostic reagent companies and healthcare providers for market expansion. |
8.4 Rate of adoption of personalized medicine and corresponding demand for specialized diagnostic reagents. |
8.5 Regulatory approvals obtained for new diagnostic reagents. |
9 France Diagnostic Reagent Market - Opportunity Assessment |
9.1 France Diagnostic Reagent Market Opportunity Assessment, By Type, 2021 & 2031F |
9.2 France Diagnostic Reagent Market Opportunity Assessment, By Application, 2021 & 2031F |
10 France Diagnostic Reagent Market - Competitive Landscape |
10.1 France Diagnostic Reagent Market Revenue Share, By Companies, 2021 |
10.2 France Diagnostic Reagent Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















