France Endoscopes Market (2025-2031) | Share, Size & Revenue, Segmentation, Value, Outlook, Companies, Trends, Analysis, Competitive Landscape, Forecast, Growth, Industry
| Product Code: ETC7218214 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
France Endoscopes Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The endoscopes import market in France saw significant growth in 2024, with top exporting countries being Japan, Malaysia, Germany, USA, and Netherlands. The market concentration, as measured by the HHI, remained high but slightly decreased from 2023 to 2024. The compound annual growth rate (CAGR) from 2020 to 2024 was a healthy 6.67%, with a notable growth rate spike of 13.98% from 2023 to 2024. This indicates a thriving market for endoscope imports in France, driven by strong contributions from key exporting nations.
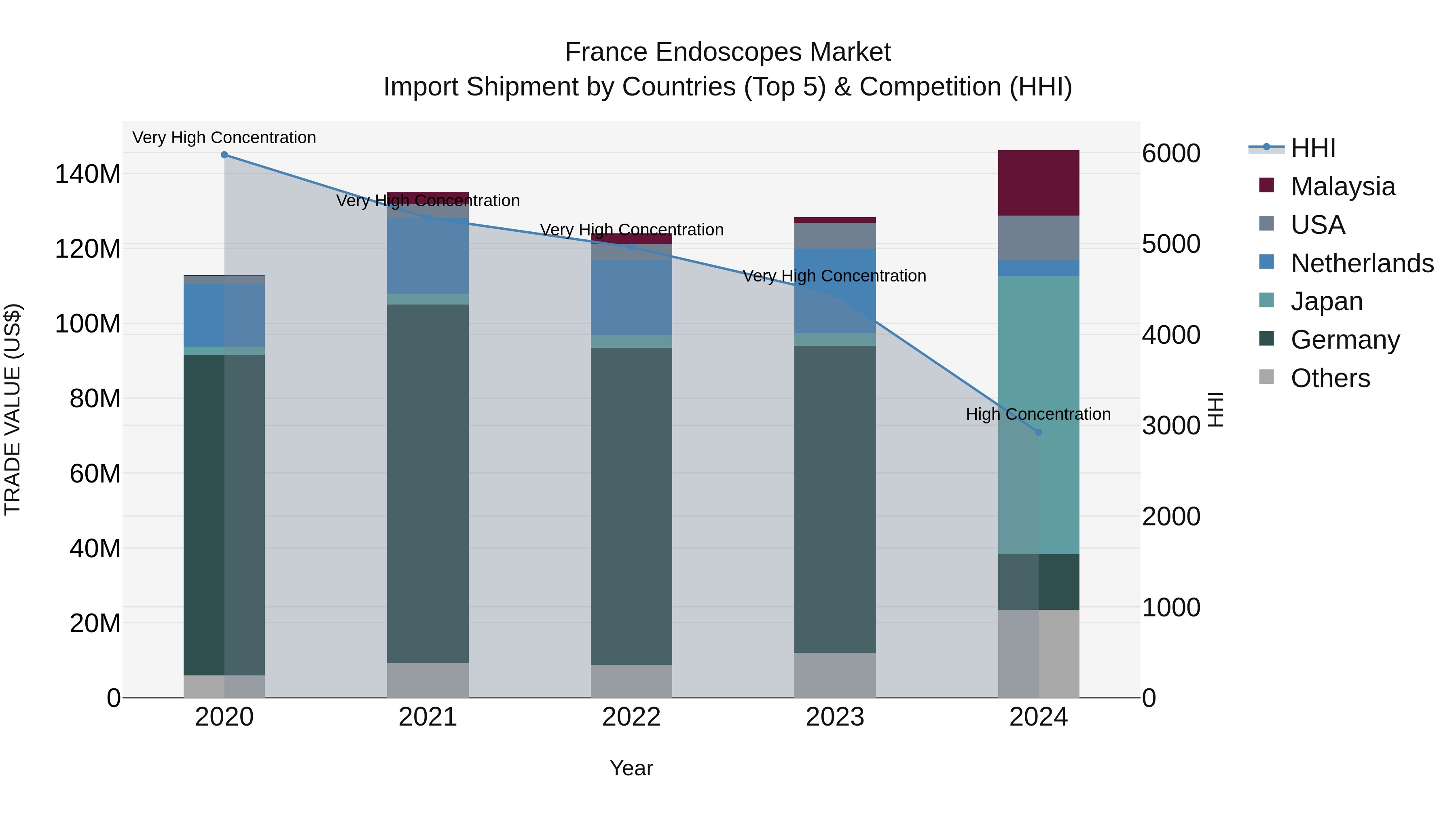
France Endoscopes Market Overview
The France Endoscopes Market is witnessing steady growth driven by the increasing prevalence of gastrointestinal disorders, technological advancements in endoscopic devices, and a growing preference for minimally invasive procedures. The market is characterized by a competitive landscape with key players such as Olympus Corporation, Karl Storz GmbH & Co. KG, and Fujifilm Holdings Corporation dominating the market share. The demand for flexible endoscopes for procedures such as colonoscopy and gastroscopy is high due to their efficacy and patient comfort. Additionally, the rising adoption of advanced endoscope accessories and imaging technologies is further propelling market growth. The increasing focus on early disease detection and treatment, along with government initiatives to improve healthcare infrastructure, are expected to drive the France Endoscopes Market in the coming years.
France Endoscopes Market Trends and Opportunities
The France Endoscopes Market is witnessing notable growth due to the increasing prevalence of gastrointestinal disorders and the rising adoption of minimally invasive surgical procedures. Technological advancements in endoscope devices, such as the integration of high-definition imaging and 3D visualization, are driving market growth. The demand for flexible endoscopes for diagnostic and therapeutic applications is also fueling market expansion. Additionally, the growing geriatric population and the rising awareness about early disease detection are creating opportunities for market players to innovate and introduce advanced endoscope products in France. Collaborations between healthcare providers and manufacturers to improve patient outcomes and reduce healthcare costs are expected to further boost market growth in the coming years.
France Endoscopes Market Challenges
In the France Endoscopes Market, some challenges include intense competition from established players, rapidly evolving technology leading to the need for constant innovation, stringent regulatory requirements, and high initial investments in research and development. Additionally, the market faces issues related to reimbursement policies and pricing pressures, as well as the need to adapt to changing healthcare policies and practices. Keeping up with advancements in endoscope technology, ensuring compliance with regulatory standards, and effectively differentiating products in a competitive market pose significant challenges for companies operating in the France Endoscopes Market. Staying ahead in terms of product development, market positioning, and addressing customer needs while managing costs and maintaining profitability are key challenges faced by industry players in this market.
France Endoscopes Market Drivers
The France Endoscopes Market is primarily being driven by technological advancements in endoscopic devices, leading to more precise and minimally invasive procedures that offer reduced patient discomfort and faster recovery times. The increasing prevalence of gastrointestinal diseases and cancers in the country is also fueling the demand for endoscopes for both diagnostic and therapeutic purposes. Additionally, the growing adoption of endoscopic procedures by healthcare facilities due to their cost-effectiveness and efficiency is boosting market growth. Furthermore, the rising awareness among patients regarding the benefits of early disease detection and treatment through endoscopy is contributing to the expanding market for endoscopic devices in France.
France Endoscopes Market Government Policy
In France, government policies related to the endoscopes market primarily focus on ensuring the safety, quality, and efficacy of medical devices, including endoscopes. The regulatory framework is overseen by the French National Agency for Medicines and Health Products Safety (ANSM) and the European Medical Devices Regulation (MDR). These regulations mandate strict requirements for the manufacturing, distribution, and use of endoscopes to protect patient safety and ensure product quality. Additionally, reimbursement policies from the French National Health Insurance system impact the market dynamics by influencing healthcare provider purchasing decisions. Compliance with these regulations and reimbursement policies is essential for companies operating in the France endoscopes market to navigate successfully and maintain market access.
France Endoscopes Market Future Outlook
The future outlook for the France Endoscopes Market appears promising, with steady growth anticipated due to increasing adoption of minimally invasive procedures, technological advancements in endoscope design, and rising prevalence of chronic diseases necessitating diagnostic and therapeutic endoscopic interventions. The market is expected to benefit from the expanding geriatric population and growing demand for early disease detection and treatment. Additionally, government initiatives aimed at improving healthcare infrastructure and accessibility to endoscopic services are likely to fuel market growth. Key players are focusing on product innovation and strategic collaborations to enhance their market presence. Overall, the France Endoscopes Market is projected to experience sustained growth over the forecast period, driven by evolving healthcare needs and advancements in medical technology.
Key Highlights of the Report:
- France Endoscopes Market Outlook
- Market Size of France Endoscopes Market, 2024
- Forecast of France Endoscopes Market, 2031
- Historical Data and Forecast of France Endoscopes Revenues & Volume for the Period 2021- 2031
- France Endoscopes Market Trend Evolution
- France Endoscopes Market Drivers and Challenges
- France Endoscopes Price Trends
- France Endoscopes Porter's Five Forces
- France Endoscopes Industry Life Cycle
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Rigid Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Laparoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Arthroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Ureteroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Cystoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Gynecology Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Endoscopes Market Revenues & Volume By Neuroendoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Flexible Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Laparoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Arthroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Ureteroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Cystoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Gynecology Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Neuroendoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Bronchoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Disposable Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Laparoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Arthroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Ureteroscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Cystoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Gynecology Endoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Neuroendoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Bronchoscopes for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By End use for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of France Endoscopes Market Revenues & Volume By Outpatient Facilities for the Period 2021- 2031
- France Endoscopes Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Flexible Endoscopes
- Market Opportunity Assessment By Disposable Endoscopes
- Market Opportunity Assessment By End use
- France Endoscopes Top Companies Market Share
- France Endoscopes Competitive Benchmarking By Technical and Operational Parameters
- France Endoscopes Company Profiles
- France Endoscopes Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
Export potential assessment - trade Analytics for 2030
Export potential enables firms to identify high-growth global markets with greater confidence by combining advanced trade intelligence with a structured quantitative methodology. The framework analyzes emerging demand trends and country-level import patterns while integrating macroeconomic and trade datasets such as GDP and population forecasts, bilateral import–export flows, tariff structures, elasticity differentials between developed and developing economies, geographic distance, and import demand projections. Using weighted trade values from 2020–2024 as the base period to project country-to-country export potential for 2030, these inputs are operationalized through calculated drivers such as gravity model parameters, tariff impact factors, and projected GDP per-capita growth. Through an analysis of hidden potentials, demand hotspots, and market conditions that are most favorable to success, this method enables firms to focus on target countries, maximize returns, and global expansion with data, backed by accuracy.
By factoring in the projected importer demand gap that is currently unmet and could be potential opportunity, it identifies the potential for the Exporter (Country) among 190 countries, against the general trade analysis, which identifies the biggest importer or exporter.
To discover high-growth global markets and optimize your business strategy:
Click Here- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Pakistan Contraceptive Implants Market (2025-2031) | Demand, Growth, Size, Share, Industry, Pricing Analysis, Competitive, Strategic Insights, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Companies, Challenges
- Sri Lanka Packaging Market (2026-2032) | Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints
- India Kids Watches Market (2026-2032) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Saudi Arabia Core Assurance Service Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Romania Uninterruptible Power Supply (UPS) Market (2026-2032) | Industry, Analysis, Revenue, Size, Forecast, Outlook, Value, Trends, Share, Growth & Companies
- Saudi Arabia Car Window Tinting Film, Paint Protection Film (PPF), and Ceramic Coating Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















