France Enteral Syringe Market (2025-2031) | Trends, Forecast, Companies, Value, Segmentation, Analysis, Size & Revenue, Competitive Landscape, Industry, Growth, Share, Outlook
| Product Code: ETC7218331 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
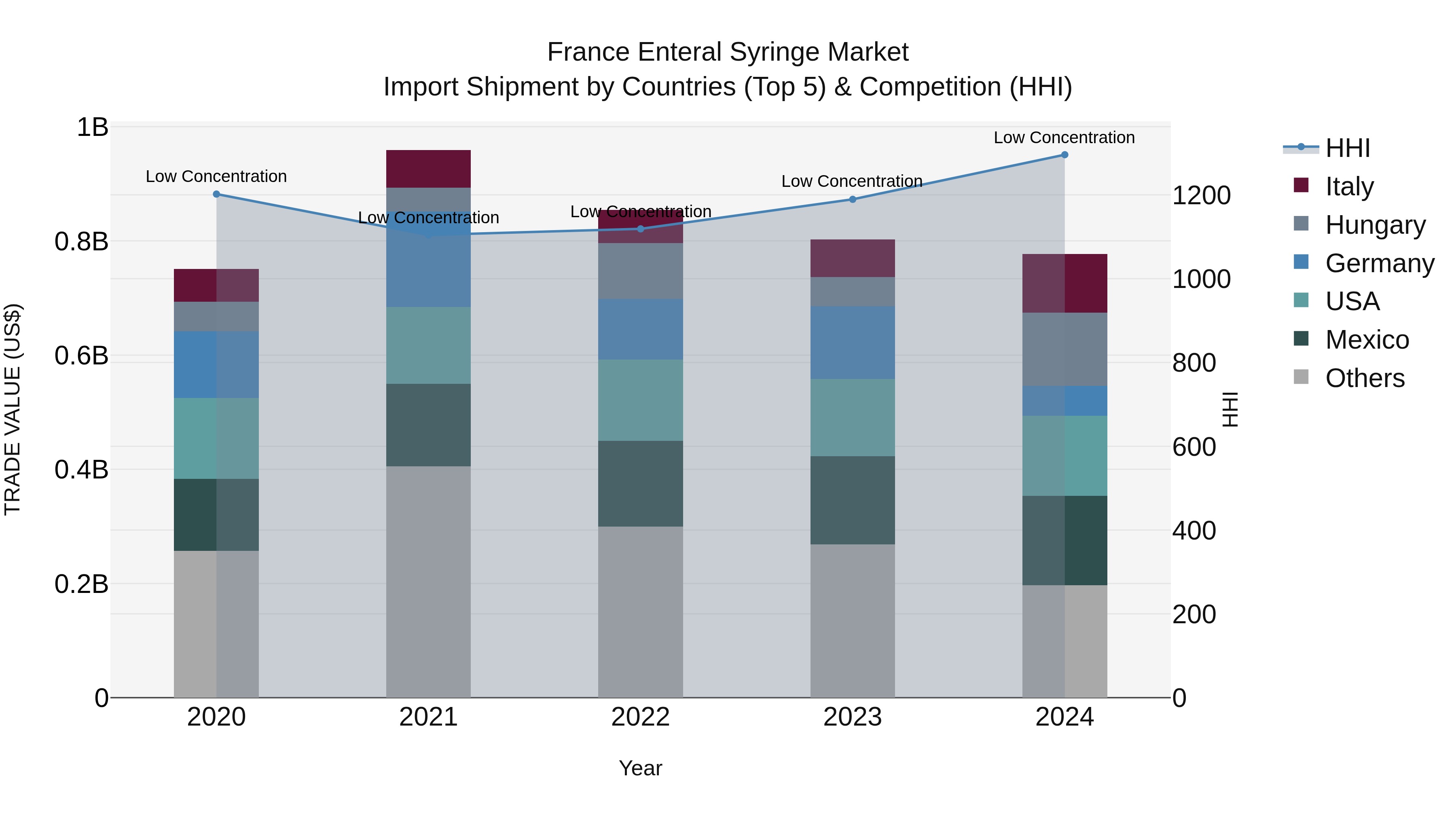
France Enteral Syringe Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s enteral syringe import market in 2024 saw significant contributions from Mexico, USA, Hungary, Italy, and Germany. Despite this diverse range of exporters, the market displayed low concentration with a Herfindahl-Hirschman Index (HHI) remaining low. The compound annual growth rate (CAGR) from 2020 to 2024 was moderate at 0.86%, although there was a slight decline in growth rate from 2023 to 2024 at -3.22%. This data indicates a stable yet slightly decelerating trend in import shipments of enteral syringes to France.
France Enteral Syringe Market Synopsis
The France Enteral Syringe market is experiencing steady growth driven by factors such as the increasing prevalence of chronic diseases, rising geriatric population, and advancements in healthcare infrastructure. Enteral syringes are widely used in hospitals, clinics, and homecare settings for the administration of liquid medications and nutrients directly into the gastrointestinal tract. The demand for enteral syringes is also supported by the growing adoption of enteral nutrition therapy as a convenient and effective method of providing essential nutrients to patients unable to consume food orally. Key players in the France Enteral Syringe market include BD, B. Braun Melsungen AG, and Cardinal Health. The market is characterized by intense competition, with companies focusing on product innovation, strategic collaborations, and geographical expansion to strengthen their market presence.
France Enteral Syringe Market Trends
The France Enteral Syringe Market is experiencing growth due to the increasing prevalence of chronic diseases and the rising geriatric population in the country. There is a growing demand for enteral syringes as they are essential for administering medications and nutrients directly into the gastrointestinal tract. Key trends in the market include the development of technologically advanced enteral syringes for more accurate dosage delivery and improved patient safety. Opportunities for market growth lie in expanding product portfolios to cater to specific patient needs, such as syringes with anti-reflux valves or ergonomic designs. Moreover, partnerships with healthcare facilities and providers to enhance distribution channels and increase market penetration could further drive the growth of the enteral syringe market in France.
France Enteral Syringe Market Challenges
In the France Enteral Syringe Market, a key challenge is the stringent regulatory requirements and standards that must be adhered to for the production and distribution of enteral syringes. This includes ensuring compliance with quality control measures, safety regulations, and certifications, which can increase production costs and lead to pricing pressures. Another challenge is the competition among manufacturers and suppliers in the market, driving the need for continuous innovation and differentiation to stay ahead. Additionally, the market may face challenges related to the adoption of enteral feeding practices by healthcare providers, as well as issues related to reimbursement policies and coverage for enteral syringes, impacting market growth and adoption rates. Overall, navigating these challenges requires a deep understanding of the regulatory landscape, market dynamics, and customer needs.
France Enteral Syringe Market Investment Opportunities
The France Enteral Syringe Market is primarily driven by factors such as the increasing prevalence of chronic diseases requiring enteral feeding, rising geriatric population, and the growing awareness about the benefits of enteral nutrition. The technological advancements in enteral syringes, such as the development of safety features and materials that enhance patient comfort, are also contributing to market growth. Additionally, the rising demand for home healthcare services and the convenience of enteral syringes for administering nutrition in non-hospital settings are further fueling market expansion. Moreover, government initiatives promoting enteral nutrition in healthcare facilities and the availability of a wide range of enteral syringes to suit different patient needs are key drivers influencing the market in France.
France Enteral Syringe Market Government Polices
The France Enteral Syringe Market is subject to several government policies aimed at ensuring patient safety and product quality. The regulatory framework for enteral syringes in France is primarily governed by the French National Agency for Medicines and Health Products Safety (ANSM) and the European Medical Device Regulation (MDR). These regulations mandate strict quality control measures, labeling requirements, and product registration processes for enteral syringes to protect patients from potential risks associated with medical devices. Additionally, reimbursement policies under the French healthcare system influence market dynamics by determining the extent of coverage for enteral syringes, which can impact pricing strategies and market access for manufacturers and suppliers operating in this sector. Compliance with these regulations is essential for market players to ensure product safety and maintain a competitive edge in the France Enteral Syringe Market.
France Enteral Syringe Market Future Outlook
The France Enteral Syringe Market is poised for steady growth in the coming years due to factors such as an aging population, increasing prevalence of chronic diseases, and advancements in enteral feeding technology. The market is expected to be driven by the rising demand for enteral feeding solutions in hospitals, long-term care facilities, and homecare settings. Additionally, the emphasis on patient comfort and safety, along with the growing adoption of enteral nutrition as a preferred mode of feeding, will further fuel market growth. Key players in the France Enteral Syringe Market are likely to focus on product innovation, strategic partnerships, and geographical expansion to capitalize on these opportunities and maintain a competitive edge in the market.
Key Highlights of the Report:
- France Enteral Syringe Market Outlook
- Market Size of France Enteral Syringe Market, 2024
- Forecast of France Enteral Syringe Market, 2031
- Historical Data and Forecast of France Enteral Syringe Revenues & Volume for the Period 2021- 2031
- France Enteral Syringe Market Trend Evolution
- France Enteral Syringe Market Drivers and Challenges
- France Enteral Syringe Price Trends
- France Enteral Syringe Porter's Five Forces
- France Enteral Syringe Industry Life Cycle
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Product Type for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Home Use Enternal Syringes for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Catheter Tip Syringes for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Single Use Enteral Syringes for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Patient Group for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Neonates for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Pediatrics for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Adults for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Oncology for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Malnutirition for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Neurological Disease for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By GI Related Disease for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By End users for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Clinics for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Home Care Settings for the Period 2021- 2031
- Historical Data and Forecast of France Enteral Syringe Market Revenues & Volume By Others for the Period 2021- 2031
- France Enteral Syringe Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Patient Group
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End users
- France Enteral Syringe Top Companies Market Share
- France Enteral Syringe Competitive Benchmarking By Technical and Operational Parameters
- France Enteral Syringe Company Profiles
- France Enteral Syringe Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Enteral Syringe Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Enteral Syringe Market Revenues & Volume, 2021 & 2031F |
3.3 France Enteral Syringe Market - Industry Life Cycle |
3.4 France Enteral Syringe Market - Porter's Five Forces |
3.5 France Enteral Syringe Market Revenues & Volume Share, By Product Type, 2021 & 2031F |
3.6 France Enteral Syringe Market Revenues & Volume Share, By Patient Group, 2021 & 2031F |
3.7 France Enteral Syringe Market Revenues & Volume Share, By Application, 2021 & 2031F |
3.8 France Enteral Syringe Market Revenues & Volume Share, By End users, 2021 & 2031F |
4 France Enteral Syringe Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of chronic diseases requiring enteral feeding |
4.2.2 Technological advancements in enteral syringe design and materials |
4.2.3 Growing elderly population in France requiring enteral nutrition support |
4.3 Market Restraints |
4.3.1 Stringent regulations and standards governing the manufacturing and distribution of enteral syringes |
4.3.2 Limited awareness and adoption of enteral feeding practices among healthcare professionals and patients |
5 France Enteral Syringe Market Trends |
6 France Enteral Syringe Market, By Types |
6.1 France Enteral Syringe Market, By Product Type |
6.1.1 Overview and Analysis |
6.1.2 France Enteral Syringe Market Revenues & Volume, By Product Type, 2021- 2031F |
6.1.3 France Enteral Syringe Market Revenues & Volume, By Home Use Enternal Syringes, 2021- 2031F |
6.1.4 France Enteral Syringe Market Revenues & Volume, By Catheter Tip Syringes, 2021- 2031F |
6.1.5 France Enteral Syringe Market Revenues & Volume, By Single Use Enteral Syringes, 2021- 2031F |
6.1.6 France Enteral Syringe Market Revenues & Volume, By Others, 2021- 2031F |
6.2 France Enteral Syringe Market, By Patient Group |
6.2.1 Overview and Analysis |
6.2.2 France Enteral Syringe Market Revenues & Volume, By Neonates, 2021- 2031F |
6.2.3 France Enteral Syringe Market Revenues & Volume, By Pediatrics, 2021- 2031F |
6.2.4 France Enteral Syringe Market Revenues & Volume, By Adults, 2021- 2031F |
6.3 France Enteral Syringe Market, By Application |
6.3.1 Overview and Analysis |
6.3.2 France Enteral Syringe Market Revenues & Volume, By Oncology, 2021- 2031F |
6.3.3 France Enteral Syringe Market Revenues & Volume, By Malnutirition, 2021- 2031F |
6.3.4 France Enteral Syringe Market Revenues & Volume, By Neurological Disease, 2021- 2031F |
6.3.5 France Enteral Syringe Market Revenues & Volume, By GI Related Disease, 2021- 2031F |
6.3.6 France Enteral Syringe Market Revenues & Volume, By Others, 2021- 2031F |
6.4 France Enteral Syringe Market, By End users |
6.4.1 Overview and Analysis |
6.4.2 France Enteral Syringe Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.4.3 France Enteral Syringe Market Revenues & Volume, By Clinics, 2021- 2031F |
6.4.4 France Enteral Syringe Market Revenues & Volume, By Home Care Settings, 2021- 2031F |
6.4.5 France Enteral Syringe Market Revenues & Volume, By Others, 2021- 2031F |
7 France Enteral Syringe Market Import-Export Trade Statistics |
7.1 France Enteral Syringe Market Export to Major Countries |
7.2 France Enteral Syringe Market Imports from Major Countries |
8 France Enteral Syringe Market Key Performance Indicators |
8.1 Adoption rate of enteral syringe usage in hospitals and healthcare facilities |
8.2 Number of enteral syringe manufacturers entering or expanding in the French market |
8.3 Research and development investments in innovative enteral syringe technologies |
9 France Enteral Syringe Market - Opportunity Assessment |
9.1 France Enteral Syringe Market Opportunity Assessment, By Product Type, 2021 & 2031F |
9.2 France Enteral Syringe Market Opportunity Assessment, By Patient Group, 2021 & 2031F |
9.3 France Enteral Syringe Market Opportunity Assessment, By Application, 2021 & 2031F |
9.4 France Enteral Syringe Market Opportunity Assessment, By End users, 2021 & 2031F |
10 France Enteral Syringe Market - Competitive Landscape |
10.1 France Enteral Syringe Market Revenue Share, By Companies, 2024 |
10.2 France Enteral Syringe Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















