France Medical Foam Market (2025-2031) Outlook | Value, Size, Companies, Revenue, Forecast, Analysis, Trends, Industry, Share & Growth
| Product Code: ETC372190 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Ravi Bhandari | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
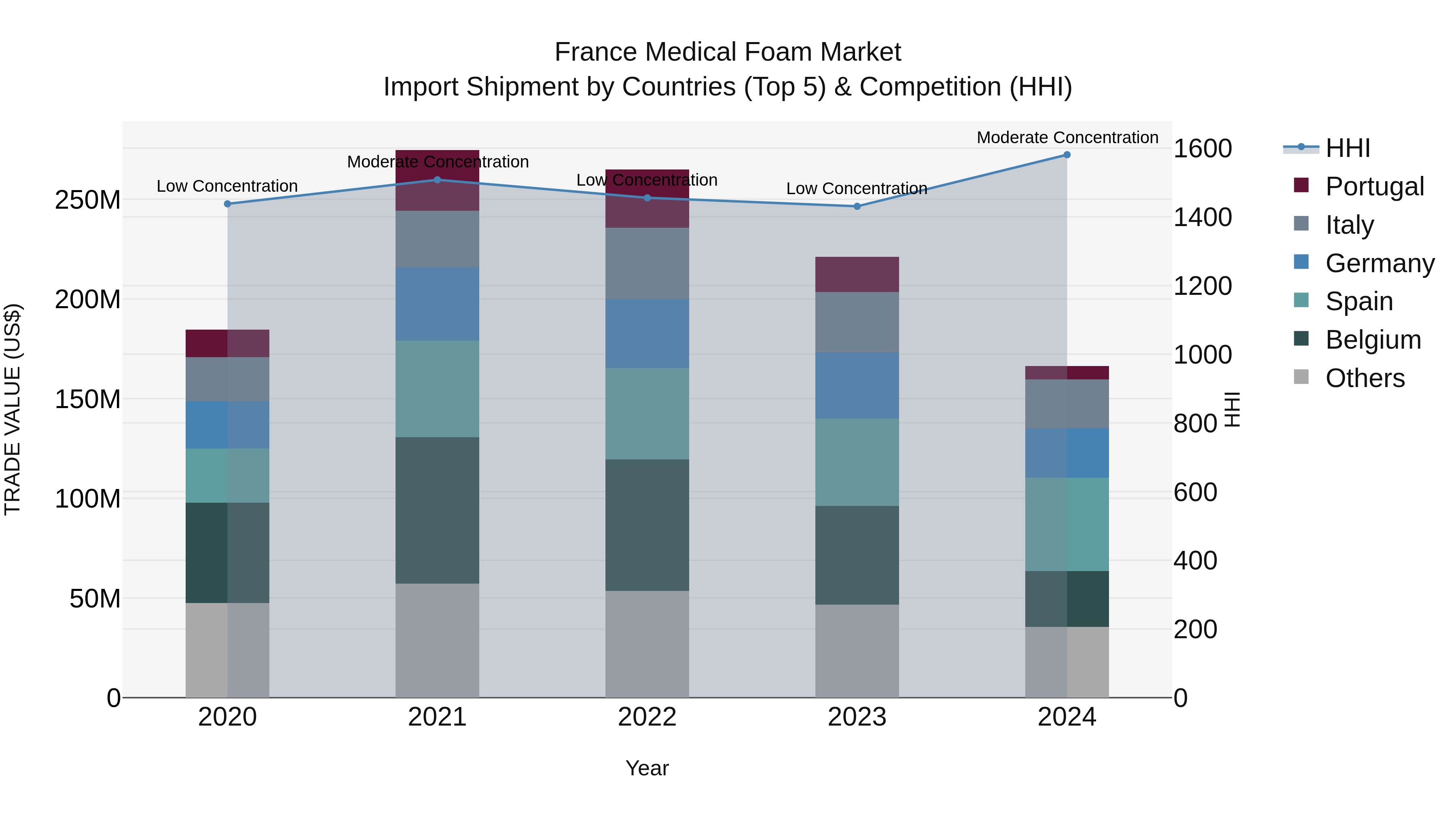
France Medical Foam Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The France medical foam import market saw a notable shift in concentration levels from low to moderate in 2024, reflecting changes in the competitive landscape. Top exporting countries including Spain, Belgium, Germany, Italy, and the UK are key players driving this market dynamic. Despite a challenging -2.56% CAGR from 2020 to 2024, the significant -24.75% growth rate in 2023-24 indicates a recent decline that may prompt strategic adjustments in the industry. Stakeholders should closely monitor these trends to capitalize on emerging opportunities and navigate potential challenges in the evolving market environment.
France Medical Foam Market Overview
France medical foam market supplies foam materials for cushioning, insulation, and wound care applications in the healthcare industry. Medical foams offer comfort, support, and protection in medical devices, mattresses, and wound dressings. With a focus on safety and performance, France medical foam manufacturers develop specialized products to meet the unique requirements of healthcare professionals and patients.
Drivers of the market
In France, the Medical Foam Market is witnessing growth driven by factors such as the demand for lightweight and cushioning materials in medical devices and furniture, the advancements in foam manufacturing technologies, and the increasing applications of foam in healthcare. Medical foam products, including foam cushions, padding, and wound dressings, offer properties such as comfort, pressure relief, and moisture management, making them suitable for applications such as medical mattresses, wheelchair cushions, and orthopedic supports. With healthcare facilities seeking to improve patient comfort, prevent pressure ulcers, and reduce infection risks, there`s a rising demand for medical foam products in the French market for healthcare furniture, medical textiles, and wound care products.
Challenges of the market
In the France medical foam market, challenges include ensuring material compatibility, performance consistency, and regulatory compliance for medical devices and cushions. Medical foam is used in applications such as wound care, orthopedic supports, and patient positioning, but its market dynamics are influenced by factors such as biocompatibility, porosity, and antimicrobial properties. Moreover, ensuring product quality and sterilizability, addressing concerns about allergic reactions, and navigating regulatory pathways for medical device approvals pose ongoing challenges for manufacturers and users in this market.
Government Policy of the market
Government regulations in France govern the production, usage, and disposal of medical foams used in healthcare applications, including standards for material safety, biocompatibility, and sterilization. Regulatory bodies ensure compliance with medical device regulations, quality standards, and patient safety requirements. Additionally, environmental regulations may address waste management and recycling practices to minimize environmental impact.
Key Highlights of the Report:
- France Medical Foam Market Outlook
- Market Size of France Medical Foam Market, 2024
- Forecast of France Medical Foam Market, 2031
- Historical Data and Forecast of France Medical Foam Revenues & Volume for the Period 2021-2031
- France Medical Foam Market Trend Evolution
- France Medical Foam Market Drivers and Challenges
- France Medical Foam Price Trends
- France Medical Foam Porter's Five Forces
- France Medical Foam Industry Life Cycle
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Product for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Flexible for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Rigid for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Spray for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Material for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Polyurethane for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Polystyrene for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Polyolefin for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Polyvinyl Chloride for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Application for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Bedding & Cushioning for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Medical Packaging for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Medical Devices & Components for the Period 2021-2031
- Historical Data and Forecast of France Medical Foam Market Revenues & Volume By Prosthetics & Wound Care for the Period 2021-2031
- France Medical Foam Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Material
- Market Opportunity Assessment By Application
- France Medical Foam Top Companies Market Share
- France Medical Foam Competitive Benchmarking By Technical and Operational Parameters
- France Medical Foam Company Profiles
- France Medical Foam Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















