France Medical Polyoxymethylene Market (2025-2031) | Outlook, Consumer Insights, Share, Drivers, Strategy, Industry, Pricing Analysis, Forecast, Segments, Opportunities, Segmentation, Growth, Companies, Investment Trends, Competition, Size, Challenges, Trends, Value, Strategic Insights, Revenue, Analysis, Competitive, Supply, Restraints, Demand
| Product Code: ETC12680905 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sachin Kumar Rai | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
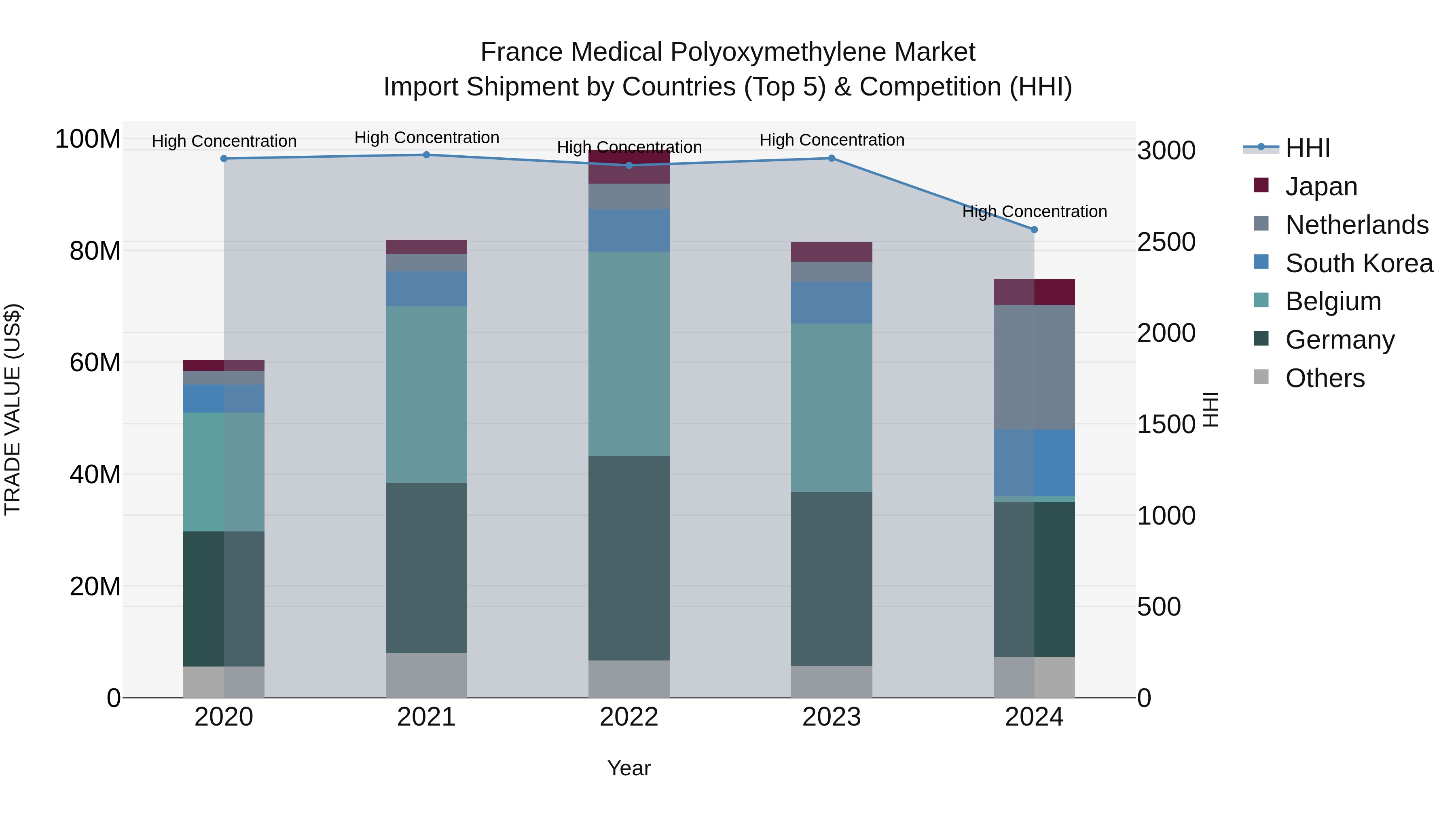
France Medical Polyoxymethylene Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, France continued to rely on key suppliers like Germany, Netherlands, South Korea, Japan, and Spain for its medical polyoxymethylene imports. Despite a slight decline in growth rate from 2023 to 2024, the compound annual growth rate (CAGR) over the period of 2020-2024 remained positive at 5.51%. The High Herfindahl-Hirschman Index (HHI) indicates a concentrated market structure, highlighting the dominance of these top exporting countries in supplying medical polyoxymethylene to France. This data suggests the importance of monitoring market dynamics and potential shifts in supply sources for this essential material in the medical industry.
France Medical Polyoxymethylene Market Overview
The medical polyoxymethylene (POM) market in France is steadily growing due to the increasing demand for high-performance plastics in the healthcare sector. POM, also known as acetal, is widely used in medical devices and equipment for its excellent mechanical properties, chemical resistance, and biocompatibility. The market is driven by the rising healthcare expenditure, technological advancements in the medical industry, and the need for lightweight and durable materials. Key players in the France medical POM market include Celanese Corporation, DuPont, and BASF SE, among others. Regulatory standards and guidelines for medical device materials are also influencing market growth. As the healthcare sector continues to evolve, the demand for medical POM is expected to increase further, presenting opportunities for market expansion and innovation.
France Medical Polyoxymethylene Market Trends
The medical polyoxymethylene market in France is experiencing a steady growth trend due to the increasing demand for medical devices and equipment in the healthcare sector. Polyoxymethylene, also known as POM or acetal, is widely used in the manufacturing of components for medical devices such as surgical instruments, catheters, and connectors due to its high strength, durability, and resistance to chemicals. Additionally, the growing focus on advanced medical technologies and the need for high-performance materials in the healthcare industry are driving the adoption of polyoxymethylene. Key players in the market are focusing on product innovation, customization, and strategic partnerships to cater to the specific requirements of the medical sector in France. Overall, the France medical polyoxymethylene market is poised for further growth in the coming years.
France Medical Polyoxymethylene Market Challenges
In the France medical polyoxymethylene market, challenges include increasing competition from alternative materials like metal and ceramics, which offer different properties and may be preferred for certain medical applications. Additionally, regulatory requirements and standards specific to medical devices can pose hurdles for manufacturers in terms of compliance and certification processes. Another challenge is the need for continuous innovation to meet the evolving demands of the healthcare industry and provide advanced solutions that address specific medical needs. Furthermore, fluctuations in raw material prices and supply chain disruptions can impact the production and availability of polyoxymethylene medical components, leading to potential delays and cost implications for market players. Overall, navigating these challenges requires industry players to adapt quickly, invest in research and development, and maintain strong relationships with regulatory bodies and supply chain partners.
France Medical Polyoxymethylene Market Investment Opportunities
In the France medical polyoxymethylene market, there are several promising investment opportunities. With the increasing demand for medical devices and equipment in the healthcare sector, the use of polyoxymethylene (POM) as a material for manufacturing components such as surgical instruments, dental implants, and prosthetic devices is gaining traction. Investors can explore opportunities in companies that specialize in the production and distribution of POM materials, as well as those involved in the development of innovative medical devices utilizing POM. Additionally, investing in research and development initiatives aimed at enhancing the properties and applications of POM in the medical field could yield long-term benefits. Overall, the France medical POM market presents a promising landscape for investors looking to capitalize on the growing healthcare industry`s demand for advanced materials.
France Medical Polyoxymethylene Market Government Policy
In France, the medical polyoxymethylene market is regulated by various government policies aimed at ensuring the safety and quality of medical devices. The French regulatory authority, Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM), oversees the approval and monitoring of medical devices, including those made from polyoxymethylene. Manufacturers are required to comply with European Union regulations such as the Medical Devices Regulation (MDR) to ensure the effectiveness and safety of their products. Additionally, French healthcare providers must adhere to guidelines on the procurement and use of medical devices to guarantee patient safety and quality of care. These policies create a framework for the medical polyoxymethylene market in France, emphasizing the importance of regulatory compliance and patient well-being.
France Medical Polyoxymethylene Market Future Outlook
The future outlook for the France medical polyoxymethylene (POM) market appears promising with steady growth expected in the coming years. The increasing demand for POM in medical devices and equipment due to its excellent mechanical properties, chemical resistance, and biocompatibility is a key driver for market expansion. Additionally, the growing healthcare industry in France, coupled with advancements in medical technology, is likely to further propel the demand for POM in various medical applications. However, factors such as stringent regulations, fluctuating raw material prices, and potential environmental concerns could pose challenges to market growth. Overall, with the continuous innovation and development of POM-based medical products, the France medical POM market is anticipated to witness positive growth opportunities in the foreseeable future.
Key Highlights of the Report:
- France Medical Polyoxymethylene Market Outlook
- Market Size of France Medical Polyoxymethylene Market,2024
- Forecast of France Medical Polyoxymethylene Market, 2031
- Historical Data and Forecast of France Medical Polyoxymethylene Revenues & Volume for the Period 2021-2031
- France Medical Polyoxymethylene Market Trend Evolution
- France Medical Polyoxymethylene Market Drivers and Challenges
- France Medical Polyoxymethylene Price Trends
- France Medical Polyoxymethylene Porter's Five Forces
- France Medical Polyoxymethylene Industry Life Cycle
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Type for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Homopolymer POM for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Copolymer POM for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Application Area for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Dialysis Machines for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Surgical Instrument Handles for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Inhalers for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Insulin Pens for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Medical Trays for the Period 2021 - 2029
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Pharmaceutical Closures for the Period 2021 - 2029
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By End User for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Hospitals for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Clinics for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Diagnostic Centers for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Product Type for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By Medical-Grade POM Resin for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By POM Rods for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By POM Sheets for the Period 2021-2031
- Historical Data and Forecast of France Medical Polyoxymethylene Market Revenues & Volume By POM Tubes for the Period 2021-2031
- France Medical Polyoxymethylene Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Application Area
- Market Opportunity Assessment By End User
- Market Opportunity Assessment By Product Type
- France Medical Polyoxymethylene Top Companies Market Share
- France Medical Polyoxymethylene Competitive Benchmarking By Technical and Operational Parameters
- France Medical Polyoxymethylene Company Profiles
- France Medical Polyoxymethylene Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Medical Polyoxymethylene Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Medical Polyoxymethylene Market Revenues & Volume, 2021 & 2031F |
3.3 France Medical Polyoxymethylene Market - Industry Life Cycle |
3.4 France Medical Polyoxymethylene Market - Porter's Five Forces |
3.5 France Medical Polyoxymethylene Market Revenues & Volume Share, By Type, 2021 & 2031F |
3.6 France Medical Polyoxymethylene Market Revenues & Volume Share, By Application Area, 2021 & 2031F |
3.7 France Medical Polyoxymethylene Market Revenues & Volume Share, By End User, 2021 & 2031F |
3.8 France Medical Polyoxymethylene Market Revenues & Volume Share, By Product Type, 2021 & 2031F |
4 France Medical Polyoxymethylene Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing demand for medical devices and equipment in France |
4.2.2 Rising focus on advanced healthcare solutions |
4.2.3 Growing adoption of polyoxymethylene in medical applications |
4.3 Market Restraints |
4.3.1 Stringent regulations and compliance requirements in the medical industry |
4.3.2 Volatility in raw material prices affecting production costs |
4.3.3 Competition from alternative materials in the medical sector |
5 France Medical Polyoxymethylene Market Trends |
6 France Medical Polyoxymethylene Market, By Types |
6.1 France Medical Polyoxymethylene Market, By Type |
6.1.1 Overview and Analysis |
6.1.2 France Medical Polyoxymethylene Market Revenues & Volume, By Type, 2021 - 2031F |
6.1.3 France Medical Polyoxymethylene Market Revenues & Volume, By Homopolymer POM, 2021 - 2031F |
6.1.4 France Medical Polyoxymethylene Market Revenues & Volume, By Copolymer POM, 2021 - 2031F |
6.2 France Medical Polyoxymethylene Market, By Application Area |
6.2.1 Overview and Analysis |
6.2.2 France Medical Polyoxymethylene Market Revenues & Volume, By Dialysis Machines, 2021 - 2031F |
6.2.3 France Medical Polyoxymethylene Market Revenues & Volume, By Surgical Instrument Handles, 2021 - 2031F |
6.2.4 France Medical Polyoxymethylene Market Revenues & Volume, By Inhalers, 2021 - 2031F |
6.2.5 France Medical Polyoxymethylene Market Revenues & Volume, By Insulin Pens, 2021 - 2031F |
6.2.6 France Medical Polyoxymethylene Market Revenues & Volume, By Medical Trays, 2021 - 2031F |
6.2.7 France Medical Polyoxymethylene Market Revenues & Volume, By Pharmaceutical Closures, 2021 - 2029F |
6.3 France Medical Polyoxymethylene Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 France Medical Polyoxymethylene Market Revenues & Volume, By Hospitals, 2021 - 2031F |
6.3.3 France Medical Polyoxymethylene Market Revenues & Volume, By Clinics, 2021 - 2031F |
6.3.4 France Medical Polyoxymethylene Market Revenues & Volume, By Diagnostic Centers, 2021 - 2031F |
6.4 France Medical Polyoxymethylene Market, By Product Type |
6.4.1 Overview and Analysis |
6.4.2 France Medical Polyoxymethylene Market Revenues & Volume, By Medical-Grade POM Resin, 2021 - 2031F |
6.4.3 France Medical Polyoxymethylene Market Revenues & Volume, By POM Rods, 2021 - 2031F |
6.4.4 France Medical Polyoxymethylene Market Revenues & Volume, By POM Sheets, 2021 - 2031F |
6.4.5 France Medical Polyoxymethylene Market Revenues & Volume, By POM Tubes, 2021 - 2031F |
7 France Medical Polyoxymethylene Market Import-Export Trade Statistics |
7.1 France Medical Polyoxymethylene Market Export to Major Countries |
7.2 France Medical Polyoxymethylene Market Imports from Major Countries |
8 France Medical Polyoxymethylene Market Key Performance Indicators |
8.1 Adoption rate of polyoxymethylene in new medical device designs |
8.2 Number of partnerships/collaborations with medical device manufacturers |
8.3 Research and development investment in enhancing polyoxymethylene properties for medical use |
9 France Medical Polyoxymethylene Market - Opportunity Assessment |
9.1 France Medical Polyoxymethylene Market Opportunity Assessment, By Type, 2021 & 2031F |
9.2 France Medical Polyoxymethylene Market Opportunity Assessment, By Application Area, 2021 & 2031F |
9.3 France Medical Polyoxymethylene Market Opportunity Assessment, By End User, 2021 & 2031F |
9.4 France Medical Polyoxymethylene Market Opportunity Assessment, By Product Type, 2021 & 2031F |
10 France Medical Polyoxymethylene Market - Competitive Landscape |
10.1 France Medical Polyoxymethylene Market Revenue Share, By Companies, 2024 |
10.2 France Medical Polyoxymethylene Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















