France MedTech Market (2025-2031) | Segments, Size, Strategic Insights, Supply, Companies, Restraints, Demand, Competition, Consumer Insights, Segmentation, Pricing Analysis, Forecast, Value, Competitive, Trends, Challenges, Drivers, Investment Trends, Outlook, Opportunities, Revenue, Strategy, Growth, Industry, Analysis, Share
| Product Code: ETC12687817 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Summon Dutta | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
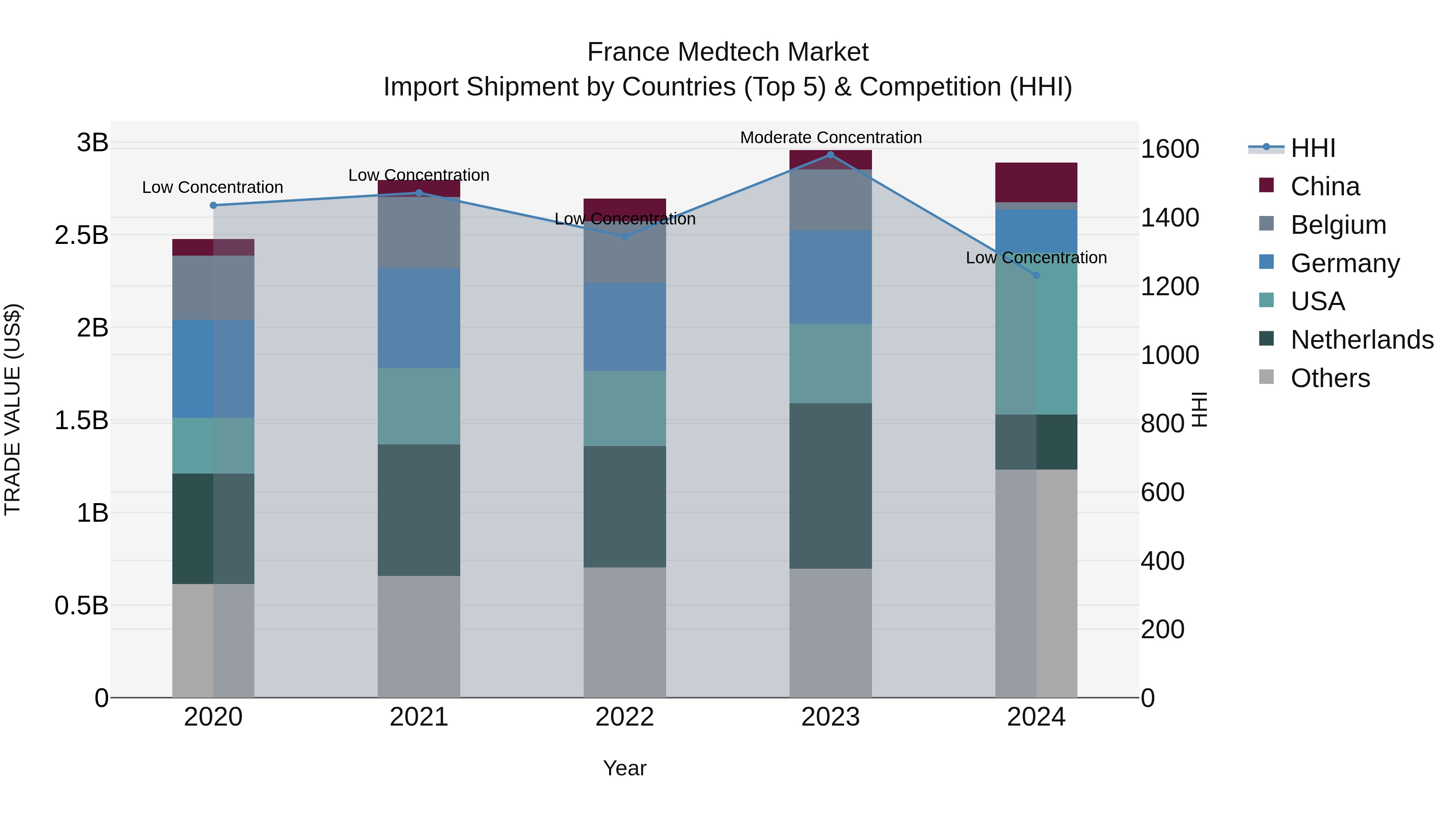
France Medtech Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s medtech import market in 2024 saw a diverse range of top exporting countries, including the USA, Netherlands, Germany, China, and Mexico. The Herfindahl-Hirschman Index (HHI) decreased from moderate to low concentration, indicating a more competitive market. Despite a slight decline in growth rate from 2023 to 2024, the compound annual growth rate (CAGR) for the period 2020-2024 remained positive at 3.94%. This suggests a stable and growing market for medtech imports in France, with opportunities for further expansion and innovation in the coming years.
France Medtech Market Overview
The France medtech market is a dynamic and innovative sector characterized by a strong emphasis on research and development. With a well-established healthcare system and a supportive regulatory environment, France is a hub for medical technology companies, particularly in the fields of imaging, diagnostics, and digital health. Key players in the market include major multinational corporations as well as a growing number of innovative startups. The market benefits from a skilled workforce, strong infrastructure, and a high level of healthcare expenditure, driving demand for advanced medical technologies. Additionally, the increasing focus on personalized medicine and patient-centric care is driving innovation in the sector, with a growing emphasis on connected devices, artificial intelligence, and telemedicine solutions. Overall, the France medtech market presents significant opportunities for growth and investment.
France Medtech Market Trends
The France medtech market is experiencing significant growth driven by factors such as technological advancements, increasing healthcare expenditure, and a growing aging population. Key trends in the market include the rise of digital health solutions, such as telemedicine and wearable devices, to improve patient outcomes and access to healthcare services. Additionally, there is a growing focus on personalized medicine and the use of artificial intelligence and big data analytics to enhance diagnosis and treatment options. Mergers and acquisitions are also prevalent in the market as companies seek to expand their offerings and reach new markets. Overall, the France medtech market is dynamic and evolving rapidly as companies innovate to meet the changing needs of patients and healthcare providers.
France Medtech Market Challenges
In the France medtech market, some challenges faced by companies include stringent regulatory requirements, complex reimbursement procedures, and fierce competition. The regulatory landscape in France is strict, requiring medtech companies to navigate a complex process for obtaining approvals for products and ensuring compliance with evolving standards. Reimbursement processes can also be challenging, as companies must demonstrate the cost-effectiveness and clinical benefits of their products to secure coverage from the French healthcare system. Additionally, the market is highly competitive, with both domestic and international players vying for market share, making it crucial for companies to differentiate their offerings and establish strong partnerships with healthcare providers. Overall, navigating these hurdles requires a deep understanding of the market dynamics and a strategic approach to succeed in the France medtech industry.
France Medtech Market Investment Opportunities
The France medtech market offers a range of investment opportunities across various segments such as medical devices, digital health solutions, and diagnostics. With a strong healthcare system, supportive regulatory environment, and high-quality research institutions, France presents a fertile ground for innovation and growth in the medtech sector. Investors can explore opportunities in companies developing cutting-edge medical technologies, telemedicine platforms, AI-driven healthcare solutions, and personalized medicine offerings. Additionally, the increasing focus on preventive healthcare and the aging population in France create a growing market for medtech products and services. Collaborations with healthcare providers, research organizations, and government initiatives further enhance the investment landscape in the France medtech market.
France Medtech Market Government Policy
In France, the medtech market is regulated by government policies aimed at ensuring patient safety, innovation, and market access. The country has a stringent regulatory framework overseen by the National Agency for the Safety of Medicines and Health Products (ANSM) and the Health Products Pricing Committee (CEPS). Medtech companies must comply with European Union regulations such as CE marking for medical devices and undergo rigorous evaluation processes before commercialization. Additionally, the French government has implemented policies to promote innovation in the medtech sector, including research and development tax incentives and public-private partnerships. Reimbursement policies are managed by the French National Health Insurance system, which sets prices and reimbursement rates for medical devices to ensure affordability and accessibility for patients. Overall, the government policies in France aim to foster a competitive and sustainable medtech market while prioritizing patient safety and healthcare quality.
France Medtech Market Future Outlook
The future outlook for the France medtech market appears promising, driven by factors such as the increasing adoption of advanced technologies in healthcare, rising healthcare expenditure, and a growing aging population. The market is expected to experience significant growth in areas such as medical devices, digital health solutions, and telemedicine. Additionally, the French government`s initiatives to promote innovation and investment in the healthcare sector are likely to further fuel market expansion. With a strong emphasis on research and development, collaborations between industry players, and an evolving regulatory landscape favorable to medtech companies, France is poised to continue its position as a key player in the global medtech market. However, challenges such as pricing pressures and regulatory complexities may also shape the market`s trajectory in the coming years.
Key Highlights of the Report:
- France MedTech Market Outlook
- Market Size of France MedTech Market,2024
- Forecast of France MedTech Market, 2031
- Historical Data and Forecast of France MedTech Revenues & Volume for the Period 2021-2031
- France MedTech Market Trend Evolution
- France MedTech Market Drivers and Challenges
- France MedTech Price Trends
- France MedTech Porter's Five Forces
- France MedTech Industry Life Cycle
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Product Type for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Diagnostic Devices for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Therapeutic Devices for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Monitoring Devices for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Supportive Devices for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Technology Type for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Imaging Systems for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Surgical Instruments for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Wearable Health Tech for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Prosthetics for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Application Area for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Hospitals for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Clinics for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Home Care for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Rehabilitation Centers for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By End User for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Healthcare Providers for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Medical Professionals for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Patients for the Period 2021-2031
- Historical Data and Forecast of France MedTech Market Revenues & Volume By Elderly for the Period 2021-2031
- France MedTech Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Technology Type
- Market Opportunity Assessment By Application Area
- Market Opportunity Assessment By End User
- France MedTech Top Companies Market Share
- France MedTech Competitive Benchmarking By Technical and Operational Parameters
- France MedTech Company Profiles
- France MedTech Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France MedTech Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France MedTech Market Revenues & Volume, 2021 & 2031F |
3.3 France MedTech Market - Industry Life Cycle |
3.4 France MedTech Market - Porter's Five Forces |
3.5 France MedTech Market Revenues & Volume Share, By Product Type, 2021 & 2031F |
3.6 France MedTech Market Revenues & Volume Share, By Technology Type, 2021 & 2031F |
3.7 France MedTech Market Revenues & Volume Share, By Application Area, 2021 & 2031F |
3.8 France MedTech Market Revenues & Volume Share, By End User, 2021 & 2031F |
4 France MedTech Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing demand for advanced healthcare technologies in France |
4.2.2 Favorable government initiatives and policies supporting the medtech industry |
4.2.3 Growing investments in research and development for innovative medical technologies |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements and compliance standards in the medtech sector |
4.3.2 Pricing pressures from healthcare providers and payers |
4.3.3 Competition from established global players in the medtech market in France |
5 France MedTech Market Trends |
6 France MedTech Market, By Types |
6.1 France MedTech Market, By Product Type |
6.1.1 Overview and Analysis |
6.1.2 France MedTech Market Revenues & Volume, By Product Type, 2021 - 2031F |
6.1.3 France MedTech Market Revenues & Volume, By Diagnostic Devices, 2021 - 2031F |
6.1.4 France MedTech Market Revenues & Volume, By Therapeutic Devices, 2021 - 2031F |
6.1.5 France MedTech Market Revenues & Volume, By Monitoring Devices, 2021 - 2031F |
6.1.6 France MedTech Market Revenues & Volume, By Supportive Devices, 2021 - 2031F |
6.2 France MedTech Market, By Technology Type |
6.2.1 Overview and Analysis |
6.2.2 France MedTech Market Revenues & Volume, By Imaging Systems, 2021 - 2031F |
6.2.3 France MedTech Market Revenues & Volume, By Surgical Instruments, 2021 - 2031F |
6.2.4 France MedTech Market Revenues & Volume, By Wearable Health Tech, 2021 - 2031F |
6.2.5 France MedTech Market Revenues & Volume, By Prosthetics, 2021 - 2031F |
6.3 France MedTech Market, By Application Area |
6.3.1 Overview and Analysis |
6.3.2 France MedTech Market Revenues & Volume, By Hospitals, 2021 - 2031F |
6.3.3 France MedTech Market Revenues & Volume, By Clinics, 2021 - 2031F |
6.3.4 France MedTech Market Revenues & Volume, By Home Care, 2021 - 2031F |
6.3.5 France MedTech Market Revenues & Volume, By Rehabilitation Centers, 2021 - 2031F |
6.4 France MedTech Market, By End User |
6.4.1 Overview and Analysis |
6.4.2 France MedTech Market Revenues & Volume, By Healthcare Providers, 2021 - 2031F |
6.4.3 France MedTech Market Revenues & Volume, By Medical Professionals, 2021 - 2031F |
6.4.4 France MedTech Market Revenues & Volume, By Patients, 2021 - 2031F |
6.4.5 France MedTech Market Revenues & Volume, By Elderly, 2021 - 2031F |
7 France MedTech Market Import-Export Trade Statistics |
7.1 France MedTech Market Export to Major Countries |
7.2 France MedTech Market Imports from Major Countries |
8 France MedTech Market Key Performance Indicators |
8.1 Adoption rate of new medical technologies in French healthcare facilities |
8.2 Number of patents filed for innovative medical devices in France |
8.3 RD expenditure by medtech companies in France |
8.4 Rate of successful commercialization of new medtech products in the French market |
9 France MedTech Market - Opportunity Assessment |
9.1 France MedTech Market Opportunity Assessment, By Product Type, 2021 & 2031F |
9.2 France MedTech Market Opportunity Assessment, By Technology Type, 2021 & 2031F |
9.3 France MedTech Market Opportunity Assessment, By Application Area, 2021 & 2031F |
9.4 France MedTech Market Opportunity Assessment, By End User, 2021 & 2031F |
10 France MedTech Market - Competitive Landscape |
10.1 France MedTech Market Revenue Share, By Companies, 2024 |
10.2 France MedTech Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
Export potential assessment - trade Analytics for 2030
Export potential enables firms to identify high-growth global markets with greater confidence by combining advanced trade intelligence with a structured quantitative methodology. The framework analyzes emerging demand trends and country-level import patterns while integrating macroeconomic and trade datasets such as GDP and population forecasts, bilateral import–export flows, tariff structures, elasticity differentials between developed and developing economies, geographic distance, and import demand projections. Using weighted trade values from 2020–2024 as the base period to project country-to-country export potential for 2030, these inputs are operationalized through calculated drivers such as gravity model parameters, tariff impact factors, and projected GDP per-capita growth. Through an analysis of hidden potentials, demand hotspots, and market conditions that are most favorable to success, this method enables firms to focus on target countries, maximize returns, and global expansion with data, backed by accuracy.
By factoring in the projected importer demand gap that is currently unmet and could be potential opportunity, it identifies the potential for the Exporter (Country) among 190 countries, against the general trade analysis, which identifies the biggest importer or exporter.
To discover high-growth global markets and optimize your business strategy:
Click Here- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- India Switchgear Market Outlook (2026 - 2032) | Size, Share, Trends, Growth, Revenue, Forecast, Analysis, Value, Outlook
- Pakistan Contraceptive Implants Market (2025-2031) | Demand, Growth, Size, Share, Industry, Pricing Analysis, Competitive, Strategic Insights, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Companies, Challenges
- Sri Lanka Packaging Market (2026-2032) | Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints
- India Kids Watches Market (2026-2032) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Saudi Arabia Core Assurance Service Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Romania Uninterruptible Power Supply (UPS) Market (2026-2032) | Industry, Analysis, Revenue, Size, Forecast, Outlook, Value, Trends, Share, Growth & Companies
- Saudi Arabia Car Window Tinting Film, Paint Protection Film (PPF), and Ceramic Coating Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















