France Orthopedic Consumables Market (2025-2031) | Segmentation, Future Prospects, Landscape, Strategic Insights, Segments, Challenges, Innovation, Share, Outlook, Drivers, Industry, Regulations, Companies, Pricing Analysis, Strategy, Consumer Insights, Market Penetration, Revenue, Size, Restraints, Competition, Trends, Value, Forecast, Growth, Supply, Opportunities, Investment Trends, Competitive Landscape, Technological Advancements, Demand, Analysis
| Product Code: ETC10739209 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
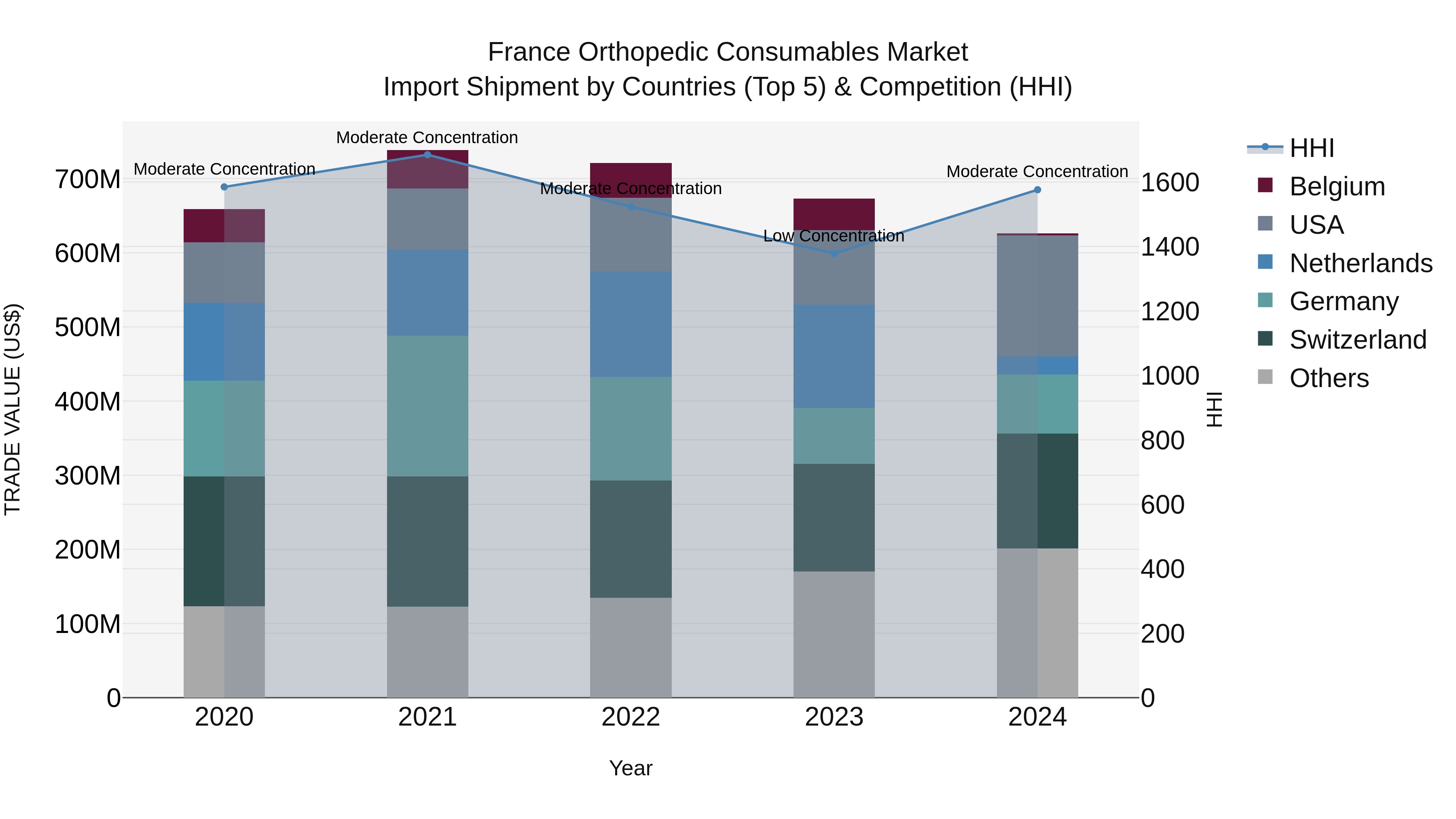
France Orthopedic Consumables Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, France saw a shift towards moderate concentration in orthopedic consumables import shipments, with top exporters being the USA, Switzerland, Germany, Mexico, and China. Despite a negative CAGR of -1.28% from 2020-2024 and a decline in growth rate of -7.02% from 2023-2024, the market continues to attract diverse sources of imports. This changing landscape suggests opportunities for market players to adapt to evolving trends and competition dynamics in the orthopedic consumables sector in France.
France Orthopedic Consumables Market Overview
The France orthopedic consumables market is experiencing steady growth, driven by an aging population, rising incidence of musculoskeletal disorders, and increased adoption of minimally invasive orthopedic procedures. Key segments include fixation consumables (such as screws, plates, pins), casting and splinting materials, and bone cements. Major players like Stryker, Zimmer Biomet, and Smith & Nephew maintain significant market share, leveraging advanced product portfolios and robust distribution channels. The French governmentâs focus on healthcare infrastructure, coupled with favorable reimbursement policies, further propels market expansion. However, the sector faces challenges such as stringent regulatory requirements, price pressures, and competition from local manufacturers. Technological advancements, including bioresorbable materials and 3D-printed implants, present new opportunities. Hospitals and specialized orthopedic centers are the primary end-users, with growing demand seen in ambulatory surgical centers. Overall, the market is poised for moderate growth, underpinned by innovation and increasing healthcare expenditure.
Trends of the Market
The France orthopedic consumables market is experiencing steady growth in 2024, driven by an aging population, rising incidence of musculoskeletal disorders, and increased adoption of minimally invasive surgical procedures. There is a notable shift towards bioabsorbable and advanced polymer-based consumables, which offer enhanced patient outcomes and reduced complication rates. Technological advancements, such as 3D-printed implants and personalized orthopedic solutions, are gaining traction, especially in trauma and sports medicine segments. Additionally, the market is witnessing a growing preference for single-use and sterilized consumables to minimize infection risks. Government initiatives supporting healthcare infrastructure and reimbursement policies are further boosting market expansion. However, cost constraints and regulatory complexities remain challenges for manufacturers. Overall, innovation, safety, and patient-centric solutions are shaping the current market landscape.
Challenges of the Market
The France Orthopedic Consumables Market faces several significant challenges. Stringent regulatory requirements slow down the approval and introduction of new products, impacting innovation and market entry. High costs associated with advanced orthopedic consumables limit access for smaller healthcare facilities and place pressure on public healthcare budgets. Reimbursement issues and pricing pressures from government policies further constrain profitability for manufacturers. Additionally, increasing competition from both domestic and international players intensifies pricing wars and reduces margins. The aging population drives demand, but also increases the prevalence of chronic conditions, complicating treatment protocols. Finally, supply chain disruptions, particularly post-pandemic, and shortages of skilled orthopedic professionals hinder efficient service delivery and market growth.
Investment Opportunities of the market
The France Orthopedic Consumables Market presents robust investment opportunities driven by the countryâs aging population, rising incidence of orthopedic disorders, and increasing adoption of advanced surgical procedures. Demand for products such as bone cements, screws, pins, and casting materials is expected to grow, fueled by government initiatives to improve healthcare infrastructure and reimbursement policies. There is significant potential in minimally invasive and bioresorbable consumables, as hospitals and clinics seek to reduce patient recovery times and complications. Strategic partnerships with local healthcare providers and leveraging digital health technologies for inventory management and patient engagement can further enhance market penetration. Additionally, compliance with stringent EU and French regulatory standards ensures high product quality, building investor confidence and fostering long-term growth prospects in this expanding sector.
Government Policy of the market
The French government regulates the orthopedic consumables market through strict compliance with EU Medical Device Regulation (MDR 2017/745), emphasizing product safety, traceability, and post-market surveillance. The Agence Nationale de Sécurité du Médicament (ANSM) oversees market authorization and quality control of orthopedic devices. Reimbursement policies are governed by the French Social Security system, requiring products to be listed on the Liste des Produits et Prestations Remboursables (LPPR), subject to cost-effectiveness assessments. Local content and public procurement laws favor innovation and sustainable manufacturing practices. Additionally, the government promotes digitalization and e-health initiatives, including the integration of orthopedic consumables within national electronic health records. These policies aim to ensure patient safety, facilitate market access for innovative products, and control healthcare spending, while aligning with broader EU directives and promoting domestic industry growth.
Future Outlook of the market
The future outlook for the France orthopedic consumables market is promising, driven by an aging population, rising prevalence of orthopedic disorders, and advancements in surgical techniques. Increasing demand for minimally invasive procedures and rapid adoption of innovative consumables such as bioabsorbable implants and bone graft substitutes are expected to fuel market growth. Government initiatives to improve healthcare infrastructure and reimbursement policies further support market expansion. However, high costs of advanced products and stringent regulatory requirements may pose challenges. Market players are likely to focus on product innovation, strategic collaborations, and expanding distribution networks to gain a competitive edge. Overall, the market is projected to experience steady growth over the next five years, with a strong emphasis on improving patient outcomes and cost-effectiveness.
Key Highlights of the Report:
- France Orthopedic Consumables Market Outlook
- Market Size of France Orthopedic Consumables Market,2024
- Forecast of France Orthopedic Consumables Market, 2031
- Historical Data and Forecast of France Orthopedic Consumables Revenues & Volume for the Period 2022-2031
- France Orthopedic Consumables Market Trend Evolution
- France Orthopedic Consumables Market Drivers and Challenges
- France Orthopedic Consumables Price Trends
- France Orthopedic Consumables Porter's Five Forces
- France Orthopedic Consumables Industry Life Cycle
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Type for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Sutures for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Bone Grafts for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Bone Substitutes for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Adhesives & Sealants for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Material Used for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Synthetic for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Natural for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Ceramic for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Biodegradable for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Application for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Soft Tissue Fixation for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Bone Reconstruction for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Trauma Surgeries for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Joint Repair for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By End User for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Hospitals for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Clinics for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Ambulatory Centers for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Research Institutes for the Period 2022-2031
- Historical Data and Forecast of France Orthopedic Consumables Market Revenues & Volume By Others for the Period 2021 - 2029
- France Orthopedic Consumables Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Material Used
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- France Orthopedic Consumables Top Companies Market Share
- France Orthopedic Consumables Competitive Benchmarking By Technical and Operational Parameters
- France Orthopedic Consumables Company Profiles
- France Orthopedic Consumables Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Orthopedic Consumables Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Orthopedic Consumables Market Revenues & Volume, 2024 & 2031F |
3.3 France Orthopedic Consumables Market - Industry Life Cycle |
3.4 France Orthopedic Consumables Market - Porter's Five Forces |
3.5 France Orthopedic Consumables Market Revenues & Volume Share, By Type, 2024 & 2031F |
3.6 France Orthopedic Consumables Market Revenues & Volume Share, By Material Used, 2024 & 2031F |
3.7 France Orthopedic Consumables Market Revenues & Volume Share, By Application, 2024 & 2031F |
3.8 France Orthopedic Consumables Market Revenues & Volume Share, By End User, 2024 & 2031F |
4 France Orthopedic Consumables Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.3 Market Restraints |
5 France Orthopedic Consumables Market Trends |
6 France Orthopedic Consumables Market, By Types |
6.1 France Orthopedic Consumables Market, By Type |
6.1.1 Overview and Analysis |
6.1.2 France Orthopedic Consumables Market Revenues & Volume, By Type, 2022 - 2031F |
6.1.3 France Orthopedic Consumables Market Revenues & Volume, By Sutures, 2022 - 2031F |
6.1.4 France Orthopedic Consumables Market Revenues & Volume, By Bone Grafts, 2022 - 2031F |
6.1.5 France Orthopedic Consumables Market Revenues & Volume, By Bone Substitutes, 2022 - 2031F |
6.1.6 France Orthopedic Consumables Market Revenues & Volume, By Adhesives & Sealants, 2022 - 2031F |
6.1.7 France Orthopedic Consumables Market Revenues & Volume, By Others, 2022 - 2031F |
6.2 France Orthopedic Consumables Market, By Material Used |
6.2.1 Overview and Analysis |
6.2.2 France Orthopedic Consumables Market Revenues & Volume, By Synthetic, 2022 - 2031F |
6.2.3 France Orthopedic Consumables Market Revenues & Volume, By Natural, 2022 - 2031F |
6.2.4 France Orthopedic Consumables Market Revenues & Volume, By Ceramic, 2022 - 2031F |
6.2.5 France Orthopedic Consumables Market Revenues & Volume, By Biodegradable, 2022 - 2031F |
6.2.6 France Orthopedic Consumables Market Revenues & Volume, By Others, 2022 - 2031F |
6.3 France Orthopedic Consumables Market, By Application |
6.3.1 Overview and Analysis |
6.3.2 France Orthopedic Consumables Market Revenues & Volume, By Soft Tissue Fixation, 2022 - 2031F |
6.3.3 France Orthopedic Consumables Market Revenues & Volume, By Bone Reconstruction, 2022 - 2031F |
6.3.4 France Orthopedic Consumables Market Revenues & Volume, By Trauma Surgeries, 2022 - 2031F |
6.3.5 France Orthopedic Consumables Market Revenues & Volume, By Joint Repair, 2022 - 2031F |
6.3.6 France Orthopedic Consumables Market Revenues & Volume, By Others, 2022 - 2031F |
6.4 France Orthopedic Consumables Market, By End User |
6.4.1 Overview and Analysis |
6.4.2 France Orthopedic Consumables Market Revenues & Volume, By Hospitals, 2022 - 2031F |
6.4.3 France Orthopedic Consumables Market Revenues & Volume, By Clinics, 2022 - 2031F |
6.4.4 France Orthopedic Consumables Market Revenues & Volume, By Ambulatory Centers, 2022 - 2031F |
6.4.5 France Orthopedic Consumables Market Revenues & Volume, By Research Institutes, 2022 - 2031F |
6.4.6 France Orthopedic Consumables Market Revenues & Volume, By Others, 2022 - 2031F |
7 France Orthopedic Consumables Market Import-Export Trade Statistics |
7.1 France Orthopedic Consumables Market Export to Major Countries |
7.2 France Orthopedic Consumables Market Imports from Major Countries |
8 France Orthopedic Consumables Market Key Performance Indicators |
9 France Orthopedic Consumables Market - Opportunity Assessment |
9.1 France Orthopedic Consumables Market Opportunity Assessment, By Type, 2024 & 2031F |
9.2 France Orthopedic Consumables Market Opportunity Assessment, By Material Used, 2024 & 2031F |
9.3 France Orthopedic Consumables Market Opportunity Assessment, By Application, 2024 & 2031F |
9.4 France Orthopedic Consumables Market Opportunity Assessment, By End User, 2024 & 2031F |
10 France Orthopedic Consumables Market - Competitive Landscape |
10.1 France Orthopedic Consumables Market Revenue Share, By Companies, 2024 |
10.2 France Orthopedic Consumables Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















