France Spine Devices Market (2025-2031) | Consumer Insights, Segments, Landscape, Competition, Regulations, Size, Share, Technological Advancements, Companies, Strategic Insights, Trends, Revenue, Value, Challenges, Competitive Landscape, Drivers, Industry, Outlook, Pricing Analysis, Market Penetration, Growth, Supply, Segmentation, Forecast, Opportunities, Restraints, Investment Trends, Strategy, Future Prospects, Analysis, Innovation, Demand
| Product Code: ETC10739977 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
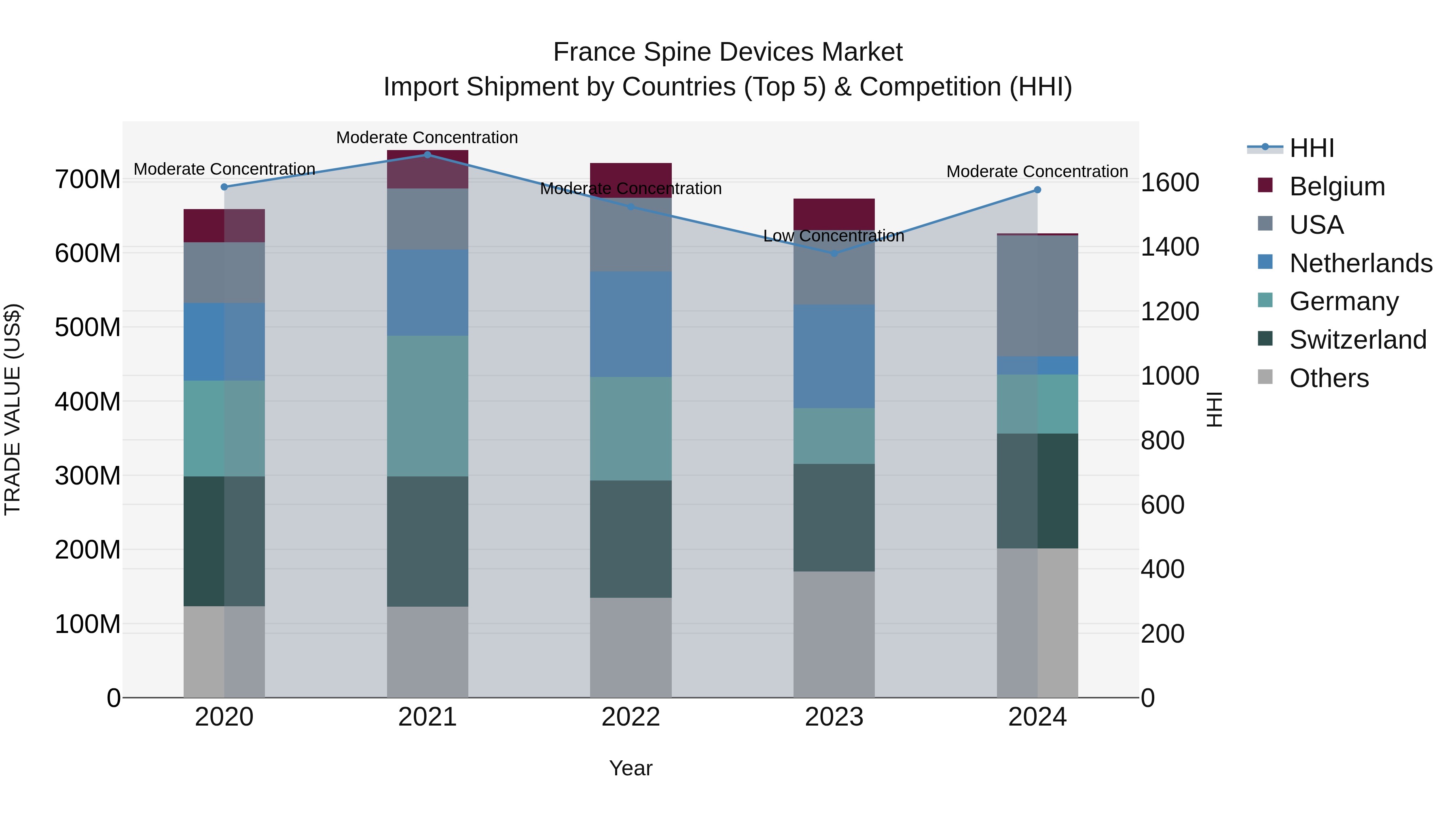
France Spine Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s spine devices import shipments in 2024 saw a shift towards moderate concentration, with top exporting countries being the USA, Switzerland, Germany, Mexico, and China. Despite a negative CAGR of -1.28% from 2020 to 2024 and a declining growth rate of -7.02% from 2023 to 2024, the market remains dynamic. The changing landscape suggests evolving market dynamics and potential opportunities for stakeholders to adapt to the shifting trends in the spine device import market in France.
France Spine Devices Market Overview
The France spine devices market is experiencing steady growth, driven by an aging population, rising incidences of spinal disorders such as degenerative disc disease and spinal stenosis, and advancements in minimally invasive surgical techniques. The market encompasses products like spinal fusion devices, non-fusion devices, artificial discs, and spinal bone stimulators. Leading global and regional companies, including Medtronic, Stryker, and Zimmer Biomet, compete alongside French manufacturers, with public and private hospitals serving as primary end-users. Increasing healthcare expenditure, favorable reimbursement policies, and growing adoption of innovative technologiesâsuch as 3D-printed implants and navigation systemsâfurther propel market expansion. However, stringent regulatory requirements, high implant costs, and concerns over long-term device safety pose challenges. The market is expected to continue its upward trajectory, supported by ongoing research, investments in healthcare infrastructure, and a shift towards outpatient and patient-specific spine procedures.
Trends of the Market
The France spine devices market is experiencing steady growth, driven by a rising prevalence of spinal disorders, an aging population, and increasing adoption of minimally invasive surgical techniques. There is a notable shift toward advanced technologies such as motion preservation devices, 3D-printed implants, and navigation-assisted surgeries, which enhance surgical precision and patient outcomes. The market is also seeing heightened demand for artificial discs and dynamic stabilization systems over traditional fusion procedures. Additionally, the expansion of outpatient spine surgery centers and favorable reimbursement policies are fostering greater accessibility to innovative treatments. Key players are investing in research and development to introduce next-generation products tailored to patient-specific needs, further propelling market expansion. However, cost constraints and stringent regulatory requirements remain key challenges for manufacturers and healthcare providers in France.
Challenges of the Market
The France Spine Devices Market faces several challenges, including stringent regulatory requirements, which can delay product approvals and market entry for innovative devices. High costs associated with advanced spine technologies and limited reimbursement policies create barriers for both manufacturers and healthcare providers. Additionally, the market is highly competitive and dominated by established international players, making it difficult for new entrants to gain significant market share. The aging population drives demand but also increases pressure on public healthcare resources, often leading to budget constraints and prioritization of cost-effective solutions. Furthermore, a shortage of skilled spine surgeons and regional disparities in access to advanced treatments hinder optimal patient outcomes and the widespread adoption of innovative spine devices. These factors collectively impact the growth and profitability of the spine devices market in France.
Investment Opportunities of the market
The France Spine Devices Market presents attractive investment opportunities driven by an aging population, rising prevalence of spinal disorders, and advancements in minimally invasive surgical techniques. Demand is particularly robust for innovative spinal implants, motion preservation devices, and navigation-assisted surgical tools that enhance patient outcomes and reduce recovery times. Furthermore, the French governmentâs emphasis on healthcare modernization and increasing adoption of digital health solutions, such as AI-driven diagnostics and robotic-assisted surgeries, are fostering a favorable environment for both established medical device firms and startups. Collaborations with hospitals and orthopedic centers to pilot new technologies, as well as investments in R&D for next-generation spinal solutions, offer high growth potential. Market entry is also facilitated by Franceâs strong regulatory framework, which supports medical innovation while ensuring patient safety, making it an appealing destination for investors seeking long-term growth in the European medtech sector.
Government Policy of the market
Government policies impacting the France spine devices market focus on stringent regulatory standards, reimbursement frameworks, and innovation support. The French National Agency for the Safety of Medicines and Health Products (ANSM) enforces strict approval and post-market surveillance for spine devices under EU MDR (Medical Device Regulation). The French healthcare system, managed by Assurance Maladie, provides reimbursement for approved spinal implants and devices, but cost-containment measures and periodic price revision can influence market accessibility. The government also encourages innovation through funding and partnerships, particularly for digital and minimally invasive solutions, as part of the national âMa Santé 2022â initiative. Additionally, procurement policies increasingly emphasize local manufacturing and sustainability. Overall, these policies aim to ensure patient safety, cost-efficiency, and the adoption of advanced medical technologies in the spine devices market.
Future Outlook of the market
The future outlook for the France Spine Devices Market is promising, driven by an aging population, rising incidences of spinal disorders, and advancements in minimally invasive surgical technologies. Increasing awareness about spine health and enhanced diagnostic capabilities are contributing to higher diagnosis and treatment rates. Additionally, government investments in healthcare infrastructure and favorable reimbursement policies are expected to boost market growth. The adoption of cutting-edge devices such as motion preservation technologies and 3D-printed implants is accelerating, with international and local players focusing on innovation and strategic partnerships. However, cost constraints and stringent regulatory requirements may pose challenges. Overall, the market is projected to experience steady growth over the next five years.
Key Highlights of the Report:
- France Spine Devices Market Outlook
- Market Size of France Spine Devices Market,2024
- Forecast of France Spine Devices Market, 2031
- Historical Data and Forecast of France Spine Devices Revenues & Volume for the Period 2022-2031
- France Spine Devices Market Trend Evolution
- France Spine Devices Market Drivers and Challenges
- France Spine Devices Price Trends
- France Spine Devices Porter's Five Forces
- France Spine Devices Industry Life Cycle
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Product Type for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Spinal Fusion Devices for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Non-Fusion Devices for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Motion Preservation Devices for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Vertebral Compression Fracture Devices for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Material Used for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Metal Alloys for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Polymer for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Ceramic for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Biodegradable for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Application for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Degenerative Disc Disease for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Spinal Stenosis for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Herniated Disc for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Spinal Deformities for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By End User for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Hospitals for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Clinics for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Ambulatory Centers for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Research Institutes for the Period 2022-2031
- Historical Data and Forecast of France Spine Devices Market Revenues & Volume By Others for the Period 2021 - 2029
- France Spine Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Material Used
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- France Spine Devices Top Companies Market Share
- France Spine Devices Competitive Benchmarking By Technical and Operational Parameters
- France Spine Devices Company Profiles
- France Spine Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Spine Devices Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Spine Devices Market Revenues & Volume, 2024 & 2031F |
3.3 France Spine Devices Market - Industry Life Cycle |
3.4 France Spine Devices Market - Porter's Five Forces |
3.5 France Spine Devices Market Revenues & Volume Share, By Product Type, 2024 & 2031F |
3.6 France Spine Devices Market Revenues & Volume Share, By Material Used, 2024 & 2031F |
3.7 France Spine Devices Market Revenues & Volume Share, By Application, 2024 & 2031F |
3.8 France Spine Devices Market Revenues & Volume Share, By End User, 2024 & 2031F |
4 France Spine Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.3 Market Restraints |
5 France Spine Devices Market Trends |
6 France Spine Devices Market, By Types |
6.1 France Spine Devices Market, By Product Type |
6.1.1 Overview and Analysis |
6.1.2 France Spine Devices Market Revenues & Volume, By Product Type, 2022 - 2031F |
6.1.3 France Spine Devices Market Revenues & Volume, By Spinal Fusion Devices, 2022 - 2031F |
6.1.4 France Spine Devices Market Revenues & Volume, By Non-Fusion Devices, 2022 - 2031F |
6.1.5 France Spine Devices Market Revenues & Volume, By Motion Preservation Devices, 2022 - 2031F |
6.1.6 France Spine Devices Market Revenues & Volume, By Vertebral Compression Fracture Devices, 2022 - 2031F |
6.1.7 France Spine Devices Market Revenues & Volume, By Others, 2022 - 2031F |
6.2 France Spine Devices Market, By Material Used |
6.2.1 Overview and Analysis |
6.2.2 France Spine Devices Market Revenues & Volume, By Metal Alloys, 2022 - 2031F |
6.2.3 France Spine Devices Market Revenues & Volume, By Polymer, 2022 - 2031F |
6.2.4 France Spine Devices Market Revenues & Volume, By Ceramic, 2022 - 2031F |
6.2.5 France Spine Devices Market Revenues & Volume, By Biodegradable, 2022 - 2031F |
6.2.6 France Spine Devices Market Revenues & Volume, By Others, 2022 - 2031F |
6.3 France Spine Devices Market, By Application |
6.3.1 Overview and Analysis |
6.3.2 France Spine Devices Market Revenues & Volume, By Degenerative Disc Disease, 2022 - 2031F |
6.3.3 France Spine Devices Market Revenues & Volume, By Spinal Stenosis, 2022 - 2031F |
6.3.4 France Spine Devices Market Revenues & Volume, By Herniated Disc, 2022 - 2031F |
6.3.5 France Spine Devices Market Revenues & Volume, By Spinal Deformities, 2022 - 2031F |
6.3.6 France Spine Devices Market Revenues & Volume, By Others, 2022 - 2031F |
6.4 France Spine Devices Market, By End User |
6.4.1 Overview and Analysis |
6.4.2 France Spine Devices Market Revenues & Volume, By Hospitals, 2022 - 2031F |
6.4.3 France Spine Devices Market Revenues & Volume, By Clinics, 2022 - 2031F |
6.4.4 France Spine Devices Market Revenues & Volume, By Ambulatory Centers, 2022 - 2031F |
6.4.5 France Spine Devices Market Revenues & Volume, By Research Institutes, 2022 - 2031F |
6.4.6 France Spine Devices Market Revenues & Volume, By Others, 2022 - 2031F |
7 France Spine Devices Market Import-Export Trade Statistics |
7.1 France Spine Devices Market Export to Major Countries |
7.2 France Spine Devices Market Imports from Major Countries |
8 France Spine Devices Market Key Performance Indicators |
9 France Spine Devices Market - Opportunity Assessment |
9.1 France Spine Devices Market Opportunity Assessment, By Product Type, 2024 & 2031F |
9.2 France Spine Devices Market Opportunity Assessment, By Material Used, 2024 & 2031F |
9.3 France Spine Devices Market Opportunity Assessment, By Application, 2024 & 2031F |
9.4 France Spine Devices Market Opportunity Assessment, By End User, 2024 & 2031F |
10 France Spine Devices Market - Competitive Landscape |
10.1 France Spine Devices Market Revenue Share, By Companies, 2024 |
10.2 France Spine Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













