France Suture Needles Market Outlook | Revenue, Industry, Growth, Companies, COVID-19 IMPACT, Trends, Value, Analysis, Share, Forecast & Size
| Product Code: ETC369790 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sachin Kumar Rai | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
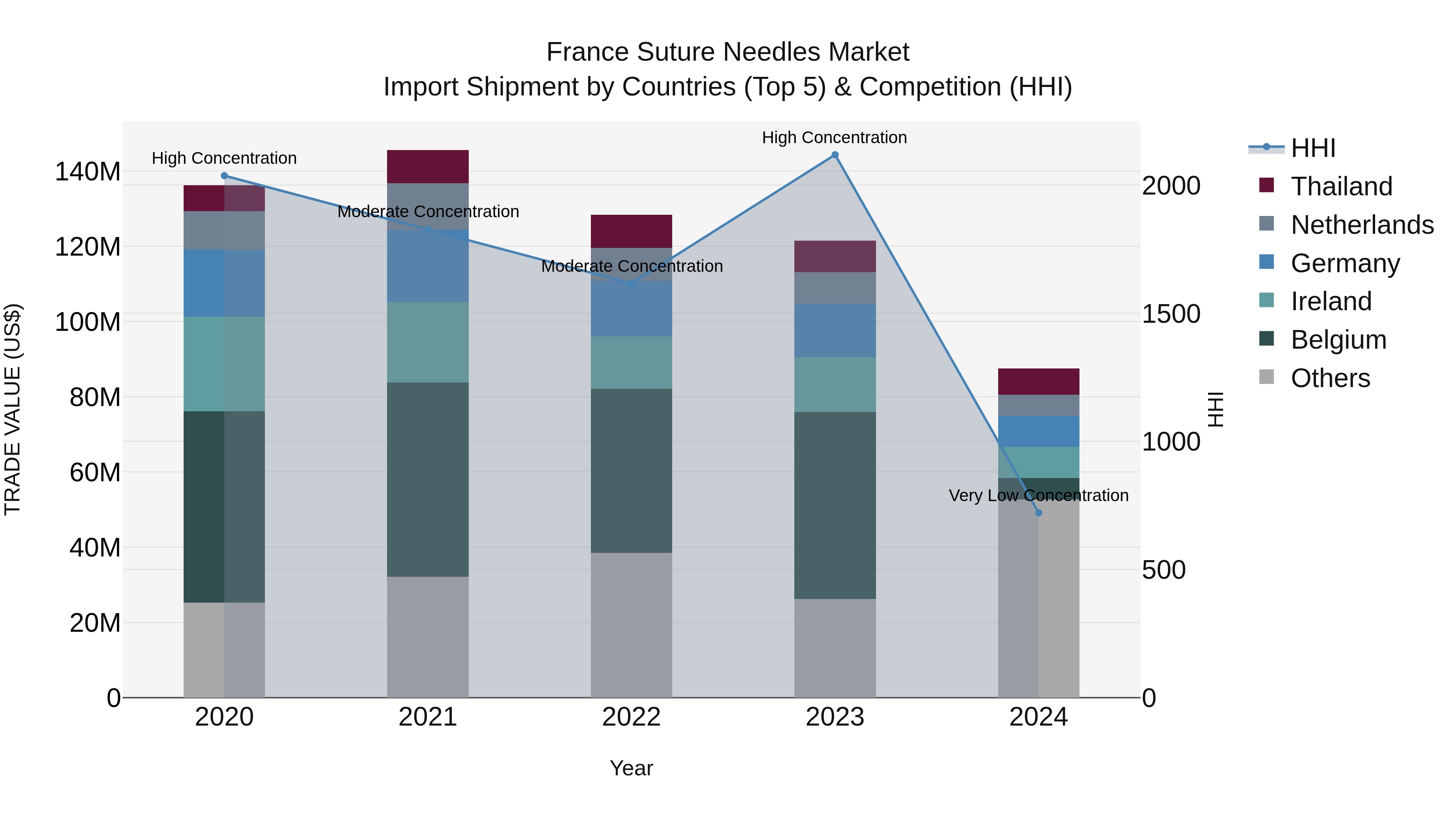
France Suture Needles Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The suture needles import market in France saw a significant shift in 2024, with top exporters being Japan, China, Ireland, Germany, and the USA. The market concentration, as measured by the HHI, dropped from high levels in 2023 to very low levels in 2024, indicating increased competition. The industry experienced a negative CAGR of -10.46% from 2020 to 2024, with a sharp decline in growth rate of -27.97% from 2023 to 2024. This dynamic market landscape suggests changing trends and challenges for suture needle importers in France.
France Suture Needles Market Synopsis
The France Suture Needles Market is a significant segment within the medical devices industry, characterized by a wide range of products catering to various medical specialties. The market is primarily driven by factors such as the increasing number of surgical procedures, advancements in healthcare infrastructure, and the rising prevalence of chronic diseases. Key players in the market offer a variety of suture needles including conventional cutting needles, taper cutting needles, and reverse cutting needles, among others. Additionally, the market is witnessing a trend towards the adoption of innovative materials for manufacturing suture needles, aimed at improving patient outcomes and reducing post-operative complications. With a focus on quality and precision, manufacturers are investing in research and development activities to introduce newer products, contributing to the overall growth of the France Suture Needles Market.
France Suture Needles Market Trends
The France suture needles market is experiencing several key trends. One prominent trend is the growing demand for advanced suture needles with improved precision and performance to enhance surgical outcomes. Manufacturers are focusing on developing innovative suture needle designs that reduce tissue trauma, improve suturing efficiency, and minimize the risk of complications. Another trend is the increasing adoption of disposable suture needles over reusable ones due to concerns regarding infection control and safety. Additionally, there is a rising preference for suture needles made from biodegradable materials to promote sustainability and reduce environmental impact. Overall, the France suture needles market is witnessing a shift towards technologically advanced, safer, and more sustainable products to meet the evolving needs of healthcare providers and patients.
France Suture Needles Market Challenges
In the France Suture Needles Market, one of the primary challenges faced is increasing competition among manufacturers and suppliers, leading to price pressures and limited profit margins. Additionally, stringent regulatory requirements and quality standards for medical devices in France pose a challenge for companies looking to enter or expand in the market. Another key challenge is the growing demand for innovative suture needle products with advanced features, which requires significant investment in research and development to stay competitive. Furthermore, the market is also influenced by changing healthcare policies, reimbursement issues, and evolving preferences of healthcare professionals, making it crucial for companies to adapt quickly to meet the dynamic needs of the market.
France Suture Needles Market Investment Opportunities
The France suture needles market presents several investment opportunities due to the increasing demand for advanced surgical procedures and the growing geriatric population in the country. Key areas for investment include the development of innovative suture needle products with enhanced features such as precision, strength, and biocompatibility to cater to the evolving needs of healthcare professionals. Additionally, investing in research and development to introduce eco-friendly and sustainable suture needles could also be a lucrative opportunity, considering the rising focus on environmental sustainability in the healthcare sector. Collaborating with healthcare institutions and surgical centers to provide training and education on the latest suture needle technologies could further enhance market penetration and revenue growth potential for investors in the France suture needles market.
Jordan Agar Market Government Policies
In France, the suture needles market is regulated by the government through various policies aimed at ensuring product safety, quality, and efficacy. The market is governed by the European Medical Devices Regulation (MDR) which sets out requirements for the design, manufacture, and marketing of medical devices, including suture needles. Manufacturers must comply with these regulations to obtain CE marking, indicating conformity with EU standards. Additionally, the French National Agency for Medicines and Health Products Safety (ANSM) oversees the market by conducting inspections, monitoring adverse events, and enforcing compliance with regulations. These policies aim to protect patient safety, promote innovation, and maintain a competitive and transparent market for suture needles in France.
France Suture Needles Market Future Outlook
The France suture needles market is expected to witness steady growth in the coming years, driven by factors such as the increasing number of surgical procedures, advancements in surgical techniques, and a growing geriatric population. The demand for suture needles is also likely to be boosted by the rising prevalence of chronic diseases and the expansion of healthcare infrastructure. Additionally, technological innovations in suture needle design, such as the development of safety needles and biodegradable materials, are anticipated to further propel market growth. However, challenges such as stringent regulations and pricing pressures may hinder market expansion to some extent. Overall, the France suture needles market is projected to experience moderate growth and opportunities for manufacturers to introduce innovative products to meet the evolving needs of healthcare professionals.
Key Highlights of the Report:
- France Suture Needles Market Outlook
- Market Size of France Suture Needles Market, 2021
- Forecast of France Suture Needles Market, 2031
- Historical Data and Forecast of France Suture Needles Revenues & Volume for the Period 2018 - 2031
- France Suture Needles Market Trend Evolution
- France Suture Needles Market Drivers and Challenges
- France Suture Needles Price Trends
- France Suture Needles Porter's Five Forces
- France Suture Needles Industry Life Cycle
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Type for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Round Bodied Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Blunt Point Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Reverse Cutting Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Conventional Cutting Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Spatula Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Tapercut Needle for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Application for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Hospital for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Clinics for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Ambulatory Surgical Centres for the Period 2018 - 2031
- Historical Data and Forecast of France Suture Needles Market Revenues & Volume By Others for the Period 2018 - 2031
- France Suture Needles Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Application
- France Suture Needles Top Companies Market Share
- France Suture Needles Competitive Benchmarking By Technical and Operational Parameters
- France Suture Needles Company Profiles
- France Suture Needles Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















