Morocco Dental Syringes Market Outlook | Companies, Share, Size, Industry, Trends, Revenue, Value, Forecast, COVID-19 IMPACT, Analysis & Growth
| Product Code: ETC362933 | Publication Date: Aug 2022 | Updated Date: Aug 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
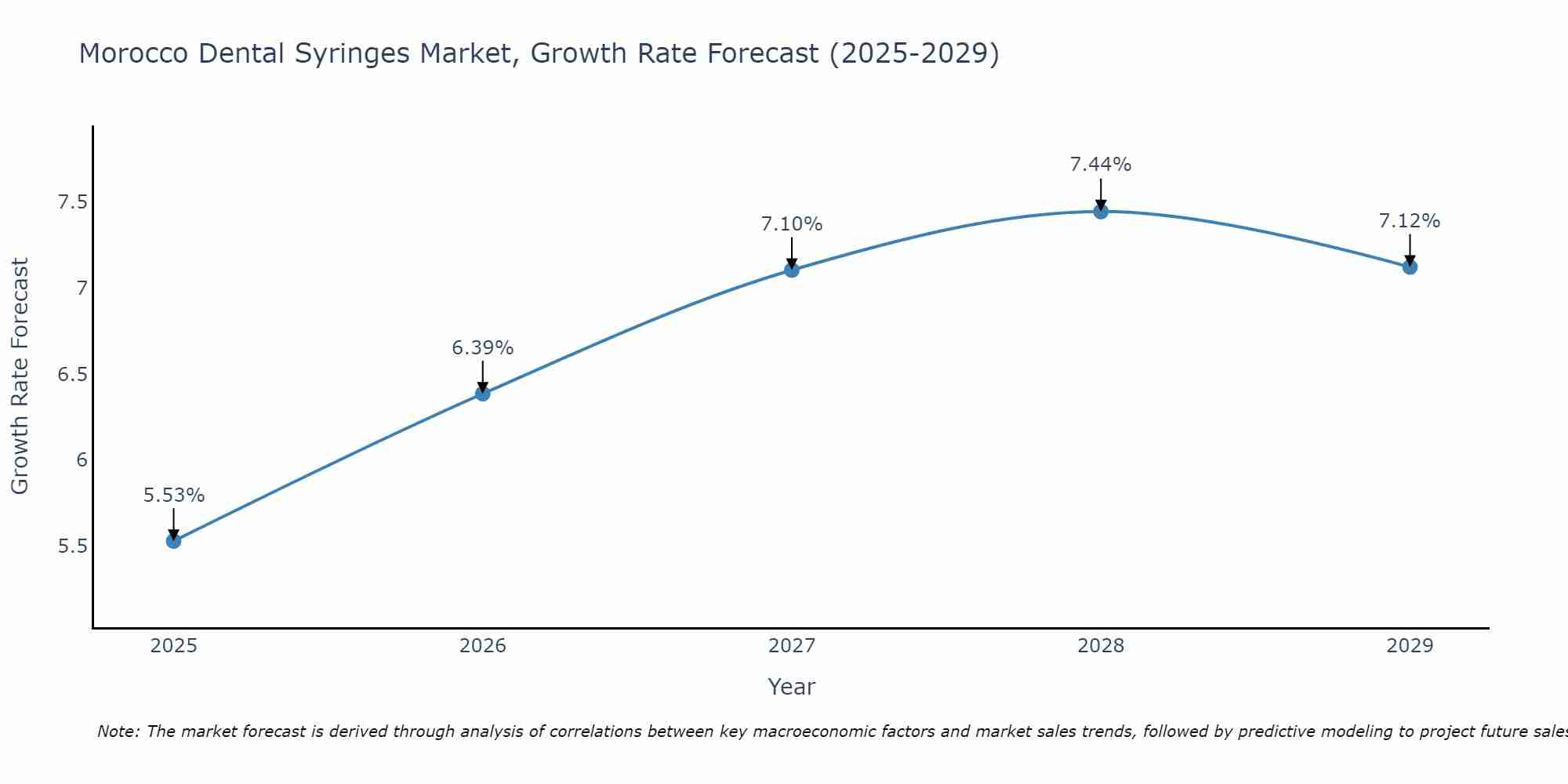
Morocco Dental Syringes Market Size Growth Rate
The Morocco Dental Syringes Market is projected to witness mixed growth rate patterns during 2025 to 2029. Growth accelerates to 7.44% in 2028, following an initial rate of 5.53%, before easing to 7.12% at the end of the period.
Dental Syringes Market: Morocco vs Top 5 Major Economies in 2027 (Africa)
Morocco's Dental Syringes market is anticipated to experience a growing growth rate of 7.10% by 2027, reflecting trends observed in the largest economy Egypt, followed by South Africa, Ethiopia, Algeria and Nigeria.

Morocco Dental Syringes Market Synopsis
The dental syringes market in Morocco is essential for delivering local anesthesia during dental procedures. The market is growing as dental practices seek reliable and safe syringe options. Advances in syringe design and materials are improving the safety and comfort of anesthesia administration.
Drivers of the market
The increasing emphasis on infection control and safety protocols in dental practices, coupled with the growing demand for precision and ease of use in dental procedures, is driving the adoption of dental syringes in Morocco. Dental syringes are essential for the delivery of local anesthesia and other dental medications, ensuring patient comfort and treatment efficacy during various dental procedures.
Challenges of the market
Challenges in the Morocco Dental Syringes Market revolve around ensuring the safety and sterility of dental syringes. Organizations face difficulties in managing the production and supply chain to maintain consistent product quality. The market is also challenged by the need to address environmental concerns related to the disposal of single-use syringes. Additionally, ensuring regulatory compliance and promoting the adoption of advanced syringe designs to improve patient comfort and reduce needle-stick injuries are crucial.
Government Policy of the market
The Morocco government recognizes the indispensable role of dental syringes in facilitating safe and effective delivery of anesthesia and other dental medications during clinical procedures. To address the various dimensions of the dental syringes market, governmental policies are being formulated to ensure product quality, safety, and accessibility. Regulatory oversight is focused on establishing standards for syringe design, materials, and sterilization processes to mitigate risks of cross-contamination and infection transmission. Moreover, efforts are underway to promote affordability, local manufacturing capabilities, and professional training to enhance the availability and utilization of dental syringes across dental practices and healthcare facilities. Collaborative endeavors between governmental health agencies, dental associations, and medical device manufacturers are encouraged to foster innovation, regulatory compliance, and equitable distribution of dental syringes, thereby safeguarding patient welfare and enhancing the quality of dental care delivery nationwide.
Key Highlights of the Report:
- Morocco Dental Syringes Market Outlook
- Market Size of Morocco Dental Syringes Market, 2024
- Forecast of Morocco Dental Syringes Market, 2031
- Historical Data and Forecast of Morocco Dental Syringes Revenues & Volume for the Period 2018 - 2031
- Morocco Dental Syringes Market Trend Evolution
- Morocco Dental Syringes Market Drivers and Challenges
- Morocco Dental Syringes Price Trends
- Morocco Dental Syringes Porter's Five Forces
- Morocco Dental Syringes Industry Life Cycle
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Product for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Reusable Dental Syringes for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Disposable Dental Syringes for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Other Products for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Type for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Aspirating Dental Syringes for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Non-aspirating Dental Syringes for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Material for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Metallic Dental Syringes for the Period 2018 - 2031
- Historical Data and Forecast of Morocco Dental Syringes Market Revenues & Volume By Plastic Dental Syringes for the Period 2018 - 2031
- Morocco Dental Syringes Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Material
- Morocco Dental Syringes Top Companies Market Share
- Morocco Dental Syringes Competitive Benchmarking By Technical and Operational Parameters
- Morocco Dental Syringes Company Profiles
- Morocco Dental Syringes Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















