Netherlands PAP and Paracetamol Market (2025-2031) | Size & Revenue, Share, Segmentation, Trends, Competitive Landscape, Outlook, Analysis, Growth, Industry, Companies, Value, Forecast
| Product Code: ETC8545040 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
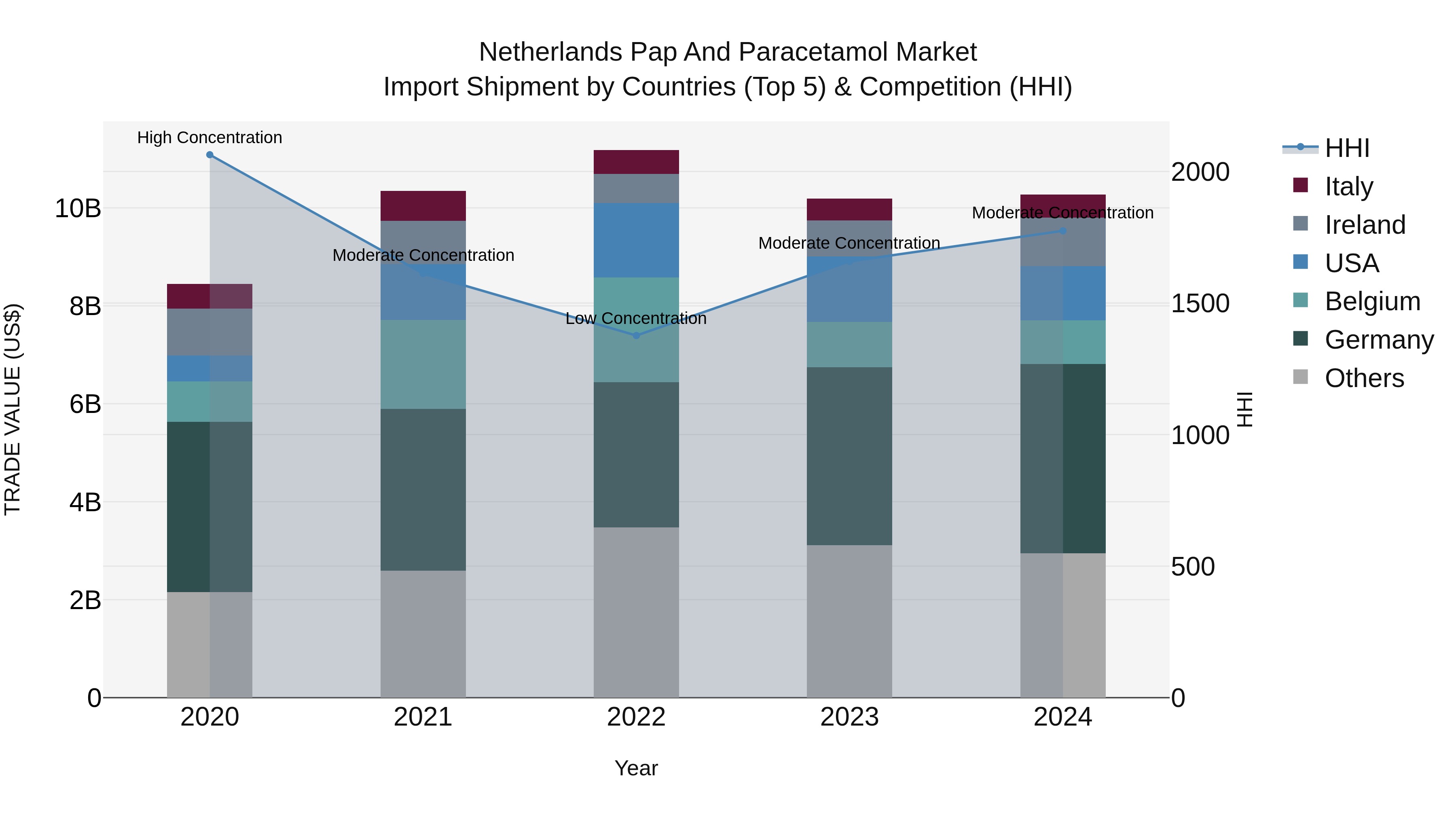
Netherlands Pap And Paracetamol Market Import Shipment by Countries (Top 5) & Competition (HHI)
The Netherlands continues to see a steady increase in pap and paracetamol import shipments, with top exporting countries in 2024 being Germany, USA, Ireland, Belgium, and Italy. The Herfindahl-Hirschman Index (HHI) indicates moderate concentration in the market in 2024, suggesting a competitive landscape. The compound annual growth rate (CAGR) from 2020 to 2024 stands at 5.02%, with a slight growth rate of 0.84% from 2023 to 2024. This data signals a stable and gradually expanding market for pap and paracetamol imports in the Netherlands.
Netherlands PAP and Paracetamol Market Synopsis
The Netherlands PAP (Phenylacetic Acid and Paracetamol) and paracetamol market are characterized by steady growth due to the high demand for pain relief medications in the country. Paracetamol, a commonly used over-the-counter medication for pain and fever relief, dominates the market due to its effectiveness and widespread availability. The market is also influenced by the increasing prevalence of chronic diseases, driving the demand for pain management products. The Netherlands has a well-established pharmaceutical industry and stringent regulatory framework, ensuring the quality and safety of PAP and paracetamol products in the market. Key players in the market focus on product innovation, strategic partnerships, and marketing efforts to maintain their market share and meet the evolving consumer needs.
Netherlands PAP and Paracetamol Market Trends
The Netherlands PAP and Paracetamol market is witnessing a trend towards increased consumer awareness and demand for over-the-counter pain relief medications, driven by a growing aging population and a focus on self-care and wellness. Opportunities exist for market players to innovate and introduce new formulations or delivery methods to cater to changing consumer preferences, such as flavored or effervescent tablets. Additionally, the rise of e-commerce platforms is providing a convenient channel for consumers to purchase these products online, presenting a significant growth opportunity for companies to expand their reach and tap into a wider customer base. Embracing digital marketing strategies and focusing on product differentiation through quality, safety, and efficacy can further enhance competitiveness in the Netherlands PAP and Paracetamol market.
Netherlands PAP and Paracetamol Market Challenges
In the Netherlands, the PAP (Prescription-Only Paracetamol) and Paracetamol market faces challenges such as increasing competition from generic brands, pricing pressure due to cost containment measures by healthcare insurers, and regulatory changes impacting the availability and pricing of these products. Additionally, concerns about the misuse and overuse of Paracetamol leading to potential liver damage have prompted stricter regulations and monitoring by authorities. Moreover, the shift towards online purchasing and e-pharmacies presents both opportunities and challenges for traditional brick-and-mortar pharmacies selling these products. Adapting to evolving consumer preferences, ensuring compliance with regulations, and maintaining competitive pricing strategies while upholding quality standards are key challenges faced by stakeholders in the Netherlands PAP and Paracetamol market.
Netherlands PAP and Paracetamol Market Investment Opportunities
The Netherlands PAP and paracetamol market is primarily driven by factors such as increasing prevalence of chronic diseases, growing geriatric population, rising consumer awareness about self-medication, and the convenience of over-the-counter availability of paracetamol products. Additionally, the country`s well-developed healthcare infrastructure, high healthcare expenditure, and strong regulatory framework also contribute to the market growth. The demand for paracetamol is further bolstered by its effectiveness in treating pain and fever, minimal side effects when taken within recommended doses, and its widespread use across various age groups. Furthermore, the expanding pharmaceutical industry in the Netherlands, coupled with advancements in drug delivery technologies, is expected to propel the market growth in the foreseeable future.
Netherlands PAP and Paracetamol Market Government Polices
The government policies related to the over-the-counter (OTC) medication market in the Netherlands, including paracetamol (PAP), are highly regulated to ensure consumer safety and quality standards. The Dutch government strictly oversees the manufacturing, distribution, and sale of OTC drugs, including paracetamol, through the Medicines Evaluation Board (MEB) and the Dutch Healthcare Inspectorate (IGJ). These regulatory bodies enforce guidelines on product labeling, dosage limits, and advertising to protect public health. Additionally, pricing policies may also be in place to prevent price gouging and ensure affordability for consumers. Market authorization for OTC medications like paracetamol requires compliance with stringent regulations, including Good Manufacturing Practices (GMP) and pharmacovigilance requirements, to maintain high standards of quality and safety in the Netherlands.
Netherlands PAP and Paracetamol Market Future Outlook
The future outlook for the Netherlands PAP (Phenylalanine and Paracetamol) and Paracetamol market appears to be positive, driven by factors such as the increasing prevalence of chronic diseases, growing aging population, and rising awareness about the benefits of these medications. The demand for PAP and Paracetamol is expected to continue to rise as they are commonly used to manage pain and fever, making them essential medications for a wide range of consumers. Additionally, the Netherlands` strong healthcare infrastructure and advanced pharmaceutical industry are likely to support market growth through innovation and product development. Overall, the PAP and Paracetamol market in the Netherlands is poised for steady expansion in the coming years, presenting opportunities for market players to capitalize on the growing demand for these essential medications.
Key Highlights of the Report:
- Netherlands PAP and Paracetamol Market Outlook
- Market Size of Netherlands PAP and Paracetamol Market, 2024
- Forecast of Netherlands PAP and Paracetamol Market, 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Revenues & Volume for the Period 2021- 2031
- Netherlands PAP and Paracetamol Market Trend Evolution
- Netherlands PAP and Paracetamol Market Drivers and Challenges
- Netherlands PAP and Paracetamol Price Trends
- Netherlands PAP and Paracetamol Porter's Five Forces
- Netherlands PAP and Paracetamol Industry Life Cycle
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Dosage Form for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Powder for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Granules for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Tablet Drug for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Granules Drug for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Oral Solutions for the Period 2021- 2031
- Historical Data and Forecast of Netherlands PAP and Paracetamol Market Revenues & Volume By Other Applications for the Period 2021- 2031
- Netherlands PAP and Paracetamol Import Export Trade Statistics
- Market Opportunity Assessment By Dosage Form
- Market Opportunity Assessment By Application
- Netherlands PAP and Paracetamol Top Companies Market Share
- Netherlands PAP and Paracetamol Competitive Benchmarking By Technical and Operational Parameters
- Netherlands PAP and Paracetamol Company Profiles
- Netherlands PAP and Paracetamol Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Netherlands PAP and Paracetamol Market Overview |
3.1 Netherlands Country Macro Economic Indicators |
3.2 Netherlands PAP and Paracetamol Market Revenues & Volume, 2021 & 2031F |
3.3 Netherlands PAP and Paracetamol Market - Industry Life Cycle |
3.4 Netherlands PAP and Paracetamol Market - Porter's Five Forces |
3.5 Netherlands PAP and Paracetamol Market Revenues & Volume Share, By Dosage Form, 2021 & 2031F |
3.6 Netherlands PAP and Paracetamol Market Revenues & Volume Share, By Application, 2021 & 2031F |
4 Netherlands PAP and Paracetamol Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing awareness about the benefits of using pap and paracetamol for pain relief and fever management |
4.2.2 Growing elderly population in the Netherlands leading to higher demand for these medications |
4.2.3 Rising prevalence of chronic diseases driving the need for regular use of pap and paracetamol |
4.3 Market Restraints |
4.3.1 Stringent regulations on the sale and distribution of over-the-counter medications in the Netherlands |
4.3.2 Presence of alternative therapies and natural remedies impacting the market share of pap and paracetamol |
4.3.3 Concerns regarding the misuse and overuse of paracetamol leading to potential health risks |
5 Netherlands PAP and Paracetamol Market Trends |
6 Netherlands PAP and Paracetamol Market, By Types |
6.1 Netherlands PAP and Paracetamol Market, By Dosage Form |
6.1.1 Overview and Analysis |
6.1.2 Netherlands PAP and Paracetamol Market Revenues & Volume, By Dosage Form, 2021- 2031F |
6.1.3 Netherlands PAP and Paracetamol Market Revenues & Volume, By Powder, 2021- 2031F |
6.1.4 Netherlands PAP and Paracetamol Market Revenues & Volume, By Granules, 2021- 2031F |
6.2 Netherlands PAP and Paracetamol Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 Netherlands PAP and Paracetamol Market Revenues & Volume, By Tablet Drug, 2021- 2031F |
6.2.3 Netherlands PAP and Paracetamol Market Revenues & Volume, By Granules Drug, 2021- 2031F |
6.2.4 Netherlands PAP and Paracetamol Market Revenues & Volume, By Oral Solutions, 2021- 2031F |
6.2.5 Netherlands PAP and Paracetamol Market Revenues & Volume, By Other Applications, 2021- 2031F |
7 Netherlands PAP and Paracetamol Market Import-Export Trade Statistics |
7.1 Netherlands PAP and Paracetamol Market Export to Major Countries |
7.2 Netherlands PAP and Paracetamol Market Imports from Major Countries |
8 Netherlands PAP and Paracetamol Market Key Performance Indicators |
8.1 Average number of prescriptions for pap and paracetamol per capita |
8.2 Percentage of pharmacies offering discounts or promotions on these medications |
8.3 Number of reported adverse events or side effects related to the use of pap and paracetamol |
8.4 Patient satisfaction scores with the effectiveness and availability of these medications |
8.5 Level of engagement and educational initiatives by healthcare providers on the proper use of pap and paracetamol |
9 Netherlands PAP and Paracetamol Market - Opportunity Assessment |
9.1 Netherlands PAP and Paracetamol Market Opportunity Assessment, By Dosage Form, 2021 & 2031F |
9.2 Netherlands PAP and Paracetamol Market Opportunity Assessment, By Application, 2021 & 2031F |
10 Netherlands PAP and Paracetamol Market - Competitive Landscape |
10.1 Netherlands PAP and Paracetamol Market Revenue Share, By Companies, 2024 |
10.2 Netherlands PAP and Paracetamol Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















