Norway Interventional Cardiology Devices Market (2025-2031) | Competitive Landscape, Trends, Share, Value, Forecast, Size & Revenue, Growth, Segmentation, Analysis, Companies, Outlook, Industry
| Product Code: ETC8670661 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
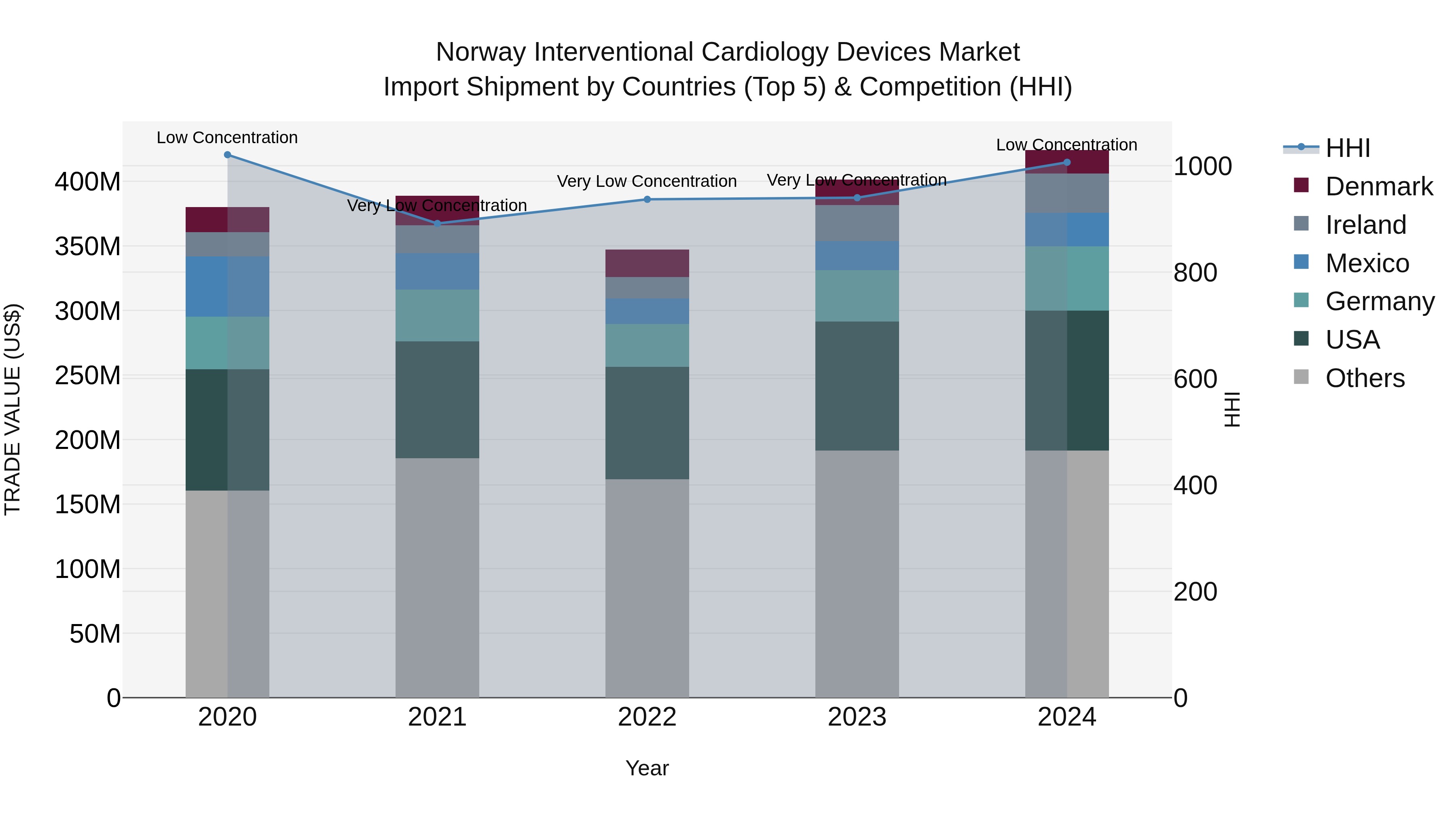
Norway Interventional Cardiology Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
Norway`s import shipments of interventional cardiology devices in 2024 saw a notable increase in growth rate compared to the previous year, with a CAGR of 2.78% from 2020 to 2024. The top exporting countries to Norway included the USA, Germany, Ireland, Mexico, and Costa Rica. The market concentration, as measured by the HHI, remained low in 2024 despite a slight increase from the previous year. This data indicates a steady and diverse flow of interventional cardiology devices into Norway, with promising opportunities for further expansion and innovation in the sector.
Norway Interventional Cardiology Devices Market Synopsis
The Norway Interventional Cardiology Devices Market is a growing sector within the country`s healthcare industry, driven by factors such as the increasing prevalence of cardiovascular diseases and a growing elderly population. The market includes a wide range of products such as coronary stents, catheters, angioplasty balloons, and imaging systems, among others. Key players in the market are focusing on developing innovative technologies to improve patient outcomes and reduce procedural complexities. The market is also witnessing a trend towards minimally invasive procedures, leading to a higher demand for interventional cardiology devices. Government initiatives to improve healthcare infrastructure and the presence of well-established healthcare facilities further contribute to the growth of the interventional cardiology devices market in Norway.
Norway Interventional Cardiology Devices Market Trends
The Norway Interventional Cardiology Devices Market is experiencing notable growth due to factors such as the increasing prevalence of cardiovascular diseases, advancements in technology leading to the development of innovative devices, and a growing elderly population. Key trends in the market include a rising demand for minimally invasive procedures, the adoption of cutting-edge imaging techniques for accurate diagnosis and treatment, and a focus on personalized medicine. Opportunities in the market lie in the expansion of product portfolios by manufacturers, strategic collaborations to enhance research and development efforts, and initiatives to improve healthcare infrastructure and accessibility. Overall, the Norway Interventional Cardiology Devices Market shows promising prospects for growth and innovation in the coming years.
Norway Interventional Cardiology Devices Market Challenges
In the Norway Interventional Cardiology Devices Market, several challenges are encountered. One significant challenge is the increasing pressure to reduce healthcare costs while maintaining high-quality care. This often leads to pricing pressures on medical device manufacturers, impacting their profitability and ability to invest in research and development. Another challenge is the stringent regulatory environment in Norway, which requires rigorous approval processes for new medical devices, leading to delays in product launches. Additionally, the market is highly competitive, with major players constantly innovating and introducing new technologies, making it challenging for smaller companies to gain market share. Overall, navigating these challenges requires companies to adapt quickly, demonstrate cost-effectiveness, and prioritize innovation to succeed in the Norway Interventional Cardiology Devices Market.
Norway Interventional Cardiology Devices Market Investment Opportunities
The Norway Interventional Cardiology Devices Market is primarily driven by factors such as the increasing prevalence of cardiovascular diseases, growing elderly population, advancements in technology leading to the development of innovative devices, and rising adoption of minimally invasive procedures. Additionally, the government initiatives promoting better healthcare infrastructure and the high healthcare expenditure in the country contribute to the market growth. The demand for interventional cardiology devices is also fueled by the rising awareness about early diagnosis and treatment of heart conditions, along with the increasing preference for cost-effective and efficient treatment options. Overall, the market is expected to continue growing due to these drivers, with a focus on improving patient outcomes and reducing healthcare costs.
Norway Interventional Cardiology Devices Market Government Polices
In Norway, the Interventional Cardiology Devices Market is regulated by the government through the Norwegian Medicines Agency (NoMA) and the Norwegian Directorate of Health. NoMA is responsible for evaluating and approving medical devices for safety and efficacy before they can be marketed in the country. The government has implemented policies to ensure the highest standards of quality and patient safety in the interventional cardiology sector. Additionally, the Norwegian healthcare system provides reimbursement for approved interventional cardiology devices, encouraging innovation and market growth. Companies operating in the Norwegian market must comply with these regulations to access the market and provide patients with high-quality, effective interventional cardiology devices.
Norway Interventional Cardiology Devices Market Future Outlook
The future outlook for the Norway Interventional Cardiology Devices Market appears promising, with a projected growth driven by factors such as the increasing prevalence of cardiovascular diseases, technological advancements in interventional cardiology procedures, and a growing elderly population. The demand for minimally invasive procedures, such as coronary angioplasty and stenting, is expected to rise, driving the market for interventional cardiology devices. Moreover, the emphasis on improving healthcare infrastructure and increasing investments in healthcare services in Norway are likely to further boost market growth. Market players are focusing on developing innovative products and expanding their product portfolios to cater to the evolving needs of healthcare professionals and patients. Overall, the Norway Interventional Cardiology Devices Market is anticipated to experience steady growth in the coming years.
Key Highlights of the Report:
- Norway Interventional Cardiology Devices Market Outlook
- Market Size of Norway Interventional Cardiology Devices Market, 2024
- Forecast of Norway Interventional Cardiology Devices Market, 2031
- Historical Data and Forecast of Norway Interventional Cardiology Devices Revenues & Volume for the Period 2021- 2031
- Norway Interventional Cardiology Devices Market Trend Evolution
- Norway Interventional Cardiology Devices Market Drivers and Challenges
- Norway Interventional Cardiology Devices Price Trends
- Norway Interventional Cardiology Devices Porter's Five Forces
- Norway Interventional Cardiology Devices Industry Life Cycle
- Historical Data and Forecast of Norway Interventional Cardiology Devices Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of Norway Interventional Cardiology Devices Market Revenues & Volume By Coronary Stents for the Period 2021- 2031
- Historical Data and Forecast of Norway Interventional Cardiology Devices Market Revenues & Volume By PTCA Balloon Catheters for the Period 2021- 2031
- Historical Data and Forecast of Norway Interventional Cardiology Devices Market Revenues & Volume By Accessory Devices for the Period 2021- 2031
- Norway Interventional Cardiology Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Norway Interventional Cardiology Devices Top Companies Market Share
- Norway Interventional Cardiology Devices Competitive Benchmarking By Technical and Operational Parameters
- Norway Interventional Cardiology Devices Company Profiles
- Norway Interventional Cardiology Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Norway Interventional Cardiology Devices Market Overview |
3.1 Norway Country Macro Economic Indicators |
3.2 Norway Interventional Cardiology Devices Market Revenues & Volume, 2021 & 2031F |
3.3 Norway Interventional Cardiology Devices Market - Industry Life Cycle |
3.4 Norway Interventional Cardiology Devices Market - Porter's Five Forces |
3.5 Norway Interventional Cardiology Devices Market Revenues & Volume Share, By Product, 2021 & 2031F |
4 Norway Interventional Cardiology Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of cardiovascular diseases in Norway |
4.2.2 Technological advancements in interventional cardiology devices |
4.2.3 Growing demand for minimally invasive procedures in cardiology |
4.3 Market Restraints |
4.3.1 Stringent regulations and approval processes for new devices |
4.3.2 High cost associated with interventional cardiology procedures |
4.3.3 Limited skilled healthcare professionals in the field of interventional cardiology |
5 Norway Interventional Cardiology Devices Market Trends |
6 Norway Interventional Cardiology Devices Market, By Types |
6.1 Norway Interventional Cardiology Devices Market, By Product |
6.1.1 Overview and Analysis |
6.1.2 Norway Interventional Cardiology Devices Market Revenues & Volume, By Product, 2021- 2031F |
6.1.3 Norway Interventional Cardiology Devices Market Revenues & Volume, By Coronary Stents, 2021- 2031F |
6.1.4 Norway Interventional Cardiology Devices Market Revenues & Volume, By PTCA Balloon Catheters, 2021- 2031F |
6.1.5 Norway Interventional Cardiology Devices Market Revenues & Volume, By Accessory Devices, 2021- 2031F |
7 Norway Interventional Cardiology Devices Market Import-Export Trade Statistics |
7.1 Norway Interventional Cardiology Devices Market Export to Major Countries |
7.2 Norway Interventional Cardiology Devices Market Imports from Major Countries |
8 Norway Interventional Cardiology Devices Market Key Performance Indicators |
8.1 Average procedure time for interventional cardiology devices |
8.2 Adoption rate of new interventional cardiology technologies |
8.3 Patient satisfaction with interventional cardiology procedures |
9 Norway Interventional Cardiology Devices Market - Opportunity Assessment |
9.1 Norway Interventional Cardiology Devices Market Opportunity Assessment, By Product, 2021 & 2031F |
10 Norway Interventional Cardiology Devices Market - Competitive Landscape |
10.1 Norway Interventional Cardiology Devices Market Revenue Share, By Companies, 2024 |
10.2 Norway Interventional Cardiology Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















