Norway Orthopedic Biomaterial Market (2025-2031) | Segmentation, Outlook, Value, Forecast, Trends, Analysis, Growth, Size & Revenue, Industry, Competitive Landscape, Share, Companies
| Product Code: ETC8674637 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sachin Kumar Rai | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Norway Orthopedic Biomaterial Market Top 5 Importing Countries and Market Competition (HHI) Analysis
Norway`s orthopedic biomaterial import market in 2024 continued to see a diverse range of suppliers, with Austria, USA, Germany, Spain, and Mexico emerging as the top exporting countries. Despite the negative CAGR of -5.65% from 2020 to 2024, the market maintained low concentration levels with a low HHI. The growth rate decline of -16.14% in 2024 indicates a challenging year for the industry, but the consistent presence of multiple suppliers offers opportunities for market resilience and potential future growth.
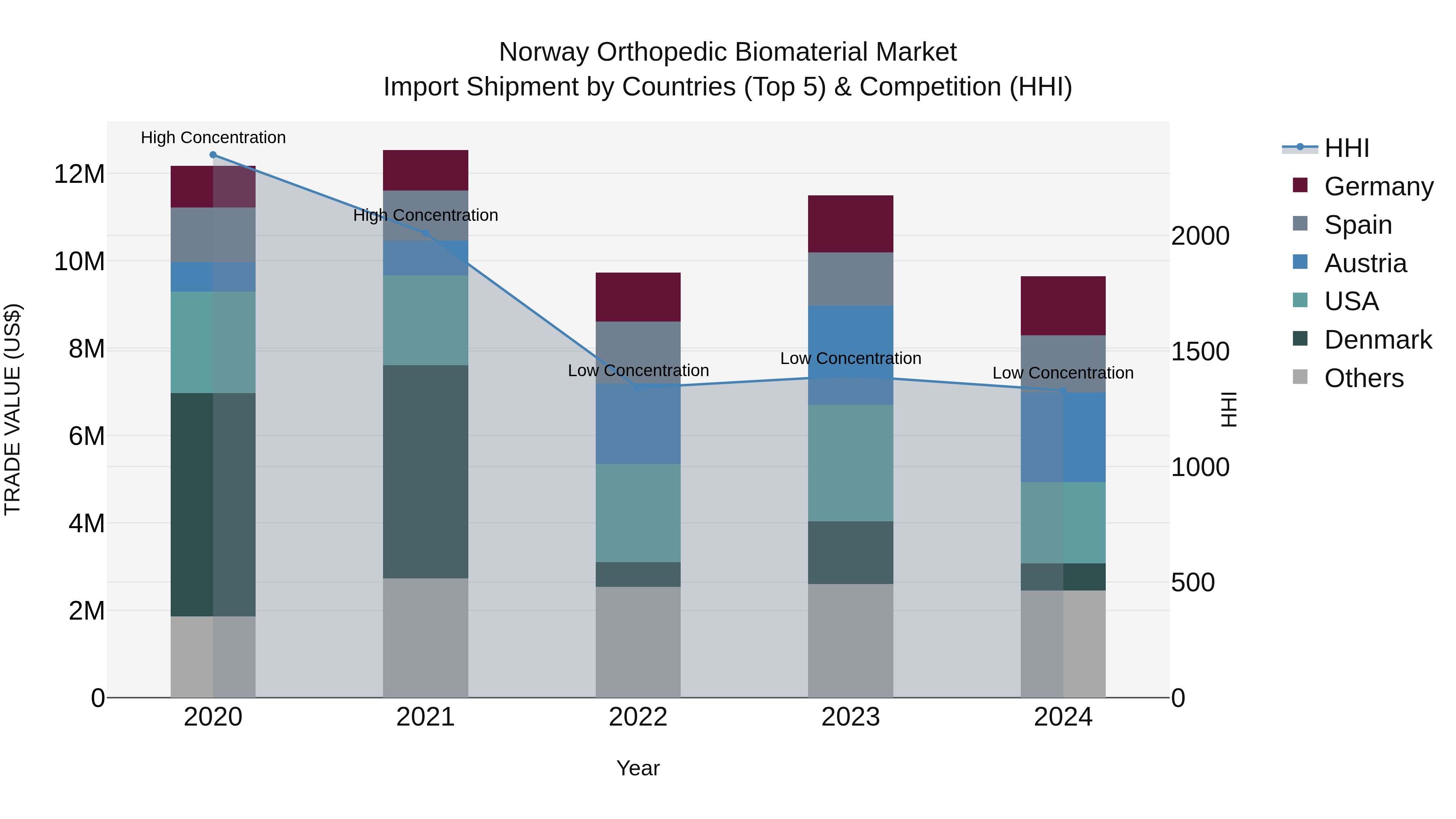
Norway Orthopedic Biomaterial Market Overview
The Norway Orthopedic Biomaterial Market is experiencing steady growth driven by factors such as an aging population, increasing prevalence of orthopedic disorders, and advancements in biomaterial technologies. The market comprises various biomaterial types including ceramics, polymers, metals, and composites used in orthopedic applications such as joint replacements, trauma fixation, and spine surgeries. Key players in the market are investing in research and development to introduce innovative biomaterial products with improved biocompatibility and performance. Government initiatives to promote the adoption of advanced orthopedic biomaterials, coupled with a rising demand for minimally invasive surgical procedures, are further propelling market growth. Overall, the Norway Orthopedic Biomaterial Market presents opportunities for market players to expand their product offerings and capitalize on the increasing demand for orthopedic solutions in the country.
Norway Orthopedic Biomaterial Market Trends and Opportunities
The Norway Orthopedic Biomaterial Market is witnessing a growing demand for advanced biomaterials due to the rising prevalence of orthopedic disorders and an aging population. Key trends include the increasing adoption of biodegradable materials, advancements in 3D printing technology for customized implants, and the development of innovative biomaterials for enhanced biocompatibility and durability. Opportunities in the market lie in the expanding applications of orthopedic biomaterials in joint replacement surgeries, spinal fusion procedures, and trauma fixation. With a strong focus on research and development, there is potential for collaborations between industry players and research institutions to drive innovation in biomaterials for orthopedic applications, ultimately improving patient outcomes and healthcare efficacy in Norway.
Norway Orthopedic Biomaterial Market Challenges
In the Norway Orthopedic Biomaterial Market, one of the key challenges is the limited availability of skilled healthcare professionals specialized in orthopedic biomaterials. This scarcity can hinder the effective adoption and utilization of advanced biomaterials in orthopedic procedures. Additionally, the high cost associated with orthopedic biomaterials poses a challenge for both healthcare providers and patients, impacting the accessibility and affordability of these innovative technologies. Moreover, regulatory complexities and stringent approval processes for orthopedic biomaterials in Norway can delay market entry for new products, creating barriers for manufacturers seeking to introduce their innovations. Overall, addressing these challenges requires strategic collaborations between industry stakeholders, healthcare institutions, and regulatory bodies to promote the growth and development of the orthopedic biomaterial market in Norway.
Norway Orthopedic Biomaterial Market Drivers
The Norway Orthopedic Biomaterial Market is primarily driven by the increasing prevalence of orthopedic disorders and injuries, leading to a rising demand for orthopedic implants and materials. The country`s aging population is also contributing to the market growth, as older individuals are more susceptible to orthopedic conditions that require surgical interventions. Technological advancements in biomaterials, such as the development of biocompatible and bioresorbable materials, are further fueling market expansion by improving patient outcomes and reducing the risk of implant rejection or complications. Additionally, growing awareness among healthcare professionals and patients regarding the benefits of orthopedic biomaterials in enhancing overall quality of life and mobility is driving adoption rates in Norway.
Norway Orthopedic Biomaterial Market Government Policies
In Norway, the Orthopedic Biomaterial Market is influenced by government policies that prioritize the use of innovative and high-quality medical devices to ensure patient safety and improve healthcare outcomes. The Norwegian Medicines Agency (NoMA) regulates the approval and monitoring of orthopedic biomaterial products to guarantee their efficacy and safety. Additionally, the Norwegian health system emphasizes cost-effectiveness and sustainability, leading to a focus on value-based procurement practices and reimbursement policies that encourage the adoption of advanced orthopedic biomaterial technologies. The government`s commitment to promoting research and development in the healthcare sector further supports the growth of the Orthopedic Biomaterial Market in Norway, fostering a competitive and dynamic industry landscape.
Norway Orthopedic Biomaterial Market Future Outlook
The Norway Orthopedic Biomaterial Market is poised for significant growth in the coming years, driven by factors such as the increasing prevalence of orthopedic disorders and injuries, advancements in biomaterial technology, and the rising elderly population. The market is expected to see a surge in demand for biomaterials used in orthopedic surgeries, such as implants, bone grafts, and scaffolds, as healthcare providers and patients seek more effective and long-lasting solutions. Additionally, the focus on personalized medicine and the development of innovative biomaterials tailored to individual patient needs will further propel market growth. With a strong healthcare infrastructure and a growing emphasis on research and development in the orthopedic field, Norway is positioned to be a key player in the global orthopedic biomaterial market in the future.
Key Highlights of the Report:
- Norway Orthopedic Biomaterial Market Outlook
- Market Size of Norway Orthopedic Biomaterial Market, 2024
- Forecast of Norway Orthopedic Biomaterial Market, 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Revenues & Volume for the Period 2021- 2031
- Norway Orthopedic Biomaterial Market Trend Evolution
- Norway Orthopedic Biomaterial Market Drivers and Challenges
- Norway Orthopedic Biomaterial Price Trends
- Norway Orthopedic Biomaterial Porter's Five Forces
- Norway Orthopedic Biomaterial Industry Life Cycle
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Type for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Metal for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Non- Metal for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Material for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Ceramics and Bioactive Glasses for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Polymers for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Calcium Phosphate Cements for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Composites for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Orthobiologics for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Orthopedic Implants for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Joint Replacement/Reconstruction for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Viscosupplementation for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Bio-Resorbable Tissue Fixation for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By End User for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Clinics for the Period 2021- 2031
- Historical Data and Forecast of Norway Orthopedic Biomaterial Market Revenues & Volume By Others for the Period 2021- 2031
- Norway Orthopedic Biomaterial Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Material
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- Norway Orthopedic Biomaterial Top Companies Market Share
- Norway Orthopedic Biomaterial Competitive Benchmarking By Technical and Operational Parameters
- Norway Orthopedic Biomaterial Company Profiles
- Norway Orthopedic Biomaterial Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Norway Orthopedic Biomaterial Market Overview |
3.1 Norway Country Macro Economic Indicators |
3.2 Norway Orthopedic Biomaterial Market Revenues & Volume, 2021 & 2031F |
3.3 Norway Orthopedic Biomaterial Market - Industry Life Cycle |
3.4 Norway Orthopedic Biomaterial Market - Porter's Five Forces |
3.5 Norway Orthopedic Biomaterial Market Revenues & Volume Share, By Type, 2021 & 2031F |
3.6 Norway Orthopedic Biomaterial Market Revenues & Volume Share, By Material, 2021 & 2031F |
3.7 Norway Orthopedic Biomaterial Market Revenues & Volume Share, By Application, 2021 & 2031F |
3.8 Norway Orthopedic Biomaterial Market Revenues & Volume Share, By End User, 2021 & 2031F |
4 Norway Orthopedic Biomaterial Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of orthopedic disorders and injuries in Norway |
4.2.2 Technological advancements in orthopedic biomaterials |
4.2.3 Growing demand for minimally invasive surgeries in orthopedics |
4.3 Market Restraints |
4.3.1 High cost associated with orthopedic biomaterial implants |
4.3.2 Stringent regulatory requirements for approval of orthopedic biomaterial products |
4.3.3 Limited reimbursement policies for orthopedic biomaterial procedures |
5 Norway Orthopedic Biomaterial Market Trends |
6 Norway Orthopedic Biomaterial Market, By Types |
6.1 Norway Orthopedic Biomaterial Market, By Type |
6.1.1 Overview and Analysis |
6.1.2 Norway Orthopedic Biomaterial Market Revenues & Volume, By Type, 2021- 2031F |
6.1.3 Norway Orthopedic Biomaterial Market Revenues & Volume, By Metal, 2021- 2031F |
6.1.4 Norway Orthopedic Biomaterial Market Revenues & Volume, By Non- Metal, 2021- 2031F |
6.2 Norway Orthopedic Biomaterial Market, By Material |
6.2.1 Overview and Analysis |
6.2.2 Norway Orthopedic Biomaterial Market Revenues & Volume, By Ceramics and Bioactive Glasses, 2021- 2031F |
6.2.3 Norway Orthopedic Biomaterial Market Revenues & Volume, By Polymers, 2021- 2031F |
6.2.4 Norway Orthopedic Biomaterial Market Revenues & Volume, By Calcium Phosphate Cements, 2021- 2031F |
6.2.5 Norway Orthopedic Biomaterial Market Revenues & Volume, By Composites, 2021- 2031F |
6.3 Norway Orthopedic Biomaterial Market, By Application |
6.3.1 Overview and Analysis |
6.3.2 Norway Orthopedic Biomaterial Market Revenues & Volume, By Orthobiologics, 2021- 2031F |
6.3.3 Norway Orthopedic Biomaterial Market Revenues & Volume, By Orthopedic Implants, 2021- 2031F |
6.3.4 Norway Orthopedic Biomaterial Market Revenues & Volume, By Joint Replacement/Reconstruction, 2021- 2031F |
6.3.5 Norway Orthopedic Biomaterial Market Revenues & Volume, By Viscosupplementation, 2021- 2031F |
6.3.6 Norway Orthopedic Biomaterial Market Revenues & Volume, By Bio-Resorbable Tissue Fixation, 2021- 2031F |
6.4 Norway Orthopedic Biomaterial Market, By End User |
6.4.1 Overview and Analysis |
6.4.2 Norway Orthopedic Biomaterial Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.4.3 Norway Orthopedic Biomaterial Market Revenues & Volume, By Clinics, 2021- 2031F |
6.4.4 Norway Orthopedic Biomaterial Market Revenues & Volume, By Others, 2021- 2031F |
7 Norway Orthopedic Biomaterial Market Import-Export Trade Statistics |
7.1 Norway Orthopedic Biomaterial Market Export to Major Countries |
7.2 Norway Orthopedic Biomaterial Market Imports from Major Countries |
8 Norway Orthopedic Biomaterial Market Key Performance Indicators |
8.1 Adoption rate of new orthopedic biomaterial technologies in Norway |
8.2 Number of orthopedic surgeries performed using biomaterial implants |
8.3 Research and development investments in orthopedic biomaterial innovations |
9 Norway Orthopedic Biomaterial Market - Opportunity Assessment |
9.1 Norway Orthopedic Biomaterial Market Opportunity Assessment, By Type, 2021 & 2031F |
9.2 Norway Orthopedic Biomaterial Market Opportunity Assessment, By Material, 2021 & 2031F |
9.3 Norway Orthopedic Biomaterial Market Opportunity Assessment, By Application, 2021 & 2031F |
9.4 Norway Orthopedic Biomaterial Market Opportunity Assessment, By End User, 2021 & 2031F |
10 Norway Orthopedic Biomaterial Market - Competitive Landscape |
10.1 Norway Orthopedic Biomaterial Market Revenue Share, By Companies, 2024 |
10.2 Norway Orthopedic Biomaterial Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















