Philippines Pediatric Perfusion Products Market (2025-2031) | Value, Trends, Analysis, Outlook, Growth, Forecast, Segmentation, Size & Revenue, Share, Companies, Competitive Landscape, Industry
| Product Code: ETC8848300 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Deep | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
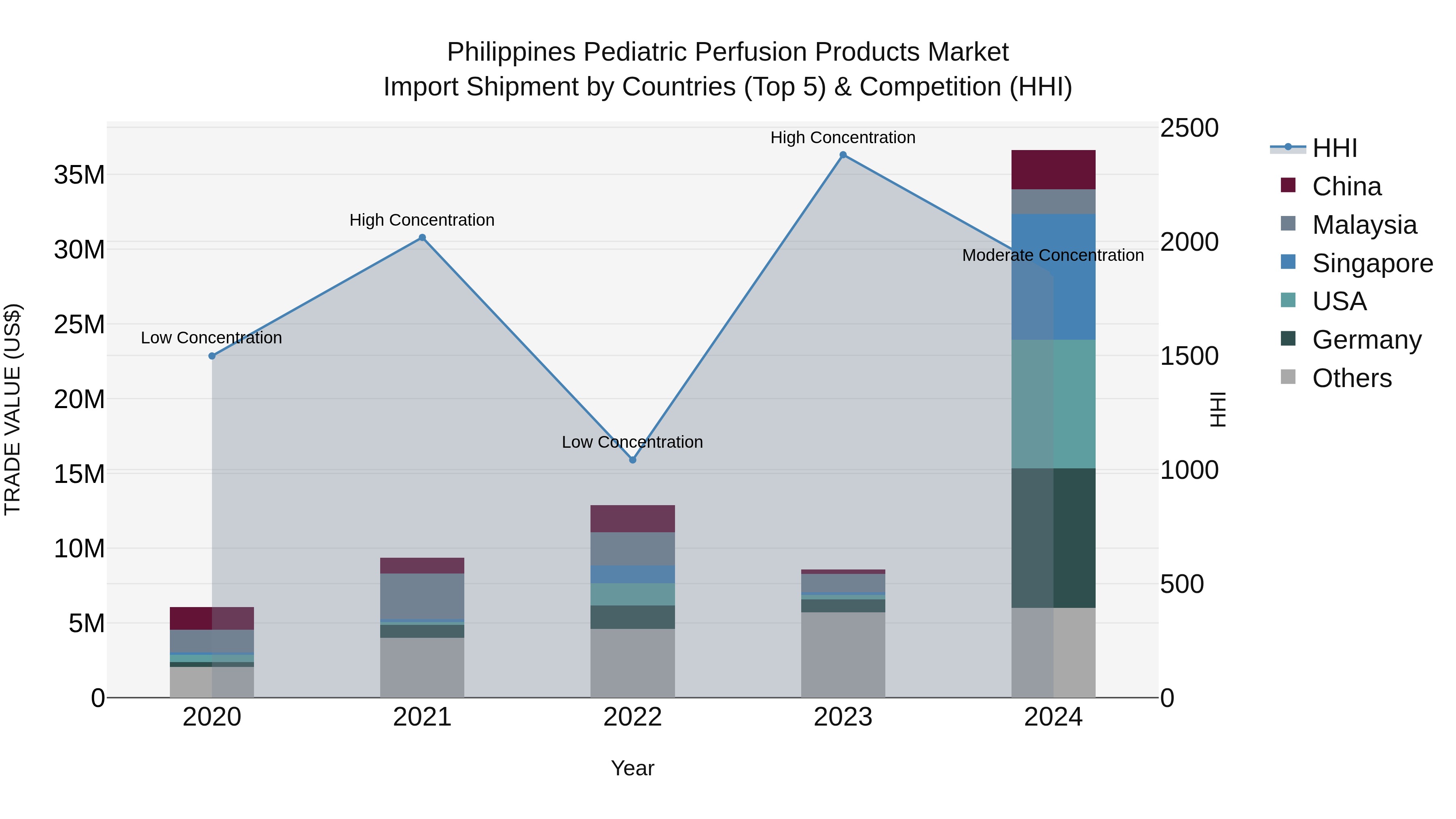
Philippines Pediatric Perfusion Products Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The pediatric perfusion products import market in the Philippines saw significant growth in 2024, with key exporting countries being Germany, USA, Singapore, China, and Japan. The market concentration, as measured by the HHI index, decreased from high to moderate in 2024, indicating a more diversified import market. The impressive compound annual growth rate (CAGR) of 56.74% from 2020 to 2024 reflects the increasing demand for pediatric perfusion products in the Philippines. Moreover, the remarkable growth rate of 327.03% from 2023 to 2024 suggests a rapid expansion in the import market for these specialized medical products.
Philippines Pediatric Perfusion Products Market Overview
The demand for pediatric perfusion products in the Philippines is driven by the increasing number of pediatric cardiac surgeries and technological innovations in extracorporeal circulation systems. Hospitals and specialized cardiac centers are investing in advanced perfusion products such as oxygenators, blood pumps, and heat exchangers to enhance surgical outcomes. Regulatory approvals and government policies promoting child healthcare further contribute to market growth. However, limited accessibility to advanced medical facilities in rural areas may restrain market development.
Drivers of the market
The pediatric perfusion products market in the Philippines is fueled by advancements in medical device technology and the increasing number of pediatric cardiac surgeries. The development of more efficient and safer perfusion devices designed specifically for children is encouraging healthcare providers to adopt these products. The growing collaboration between medical device manufacturers and healthcare institutions to improve pediatric surgical outcomes is another significant driver. Moreover, rising investments in healthcare infrastructure and the availability of government subsidies for pediatric cardiac treatments are propelling market growth.
Challenges of the market
The peanut milk market faces challenges in terms of consumer acceptance, as many people are more familiar with traditional dairy or other plant-based milks like soy or almond. Peanuts may also pose allergy risks, limiting its potential market size. Furthermore, the high cost of production, including sourcing quality peanuts and maintaining strict food safety standards, may deter local producers. Limited distribution channels and consumer awareness also contribute to slower growth in the market.
Investment opportunities in the Market
The growing adoption of advanced pediatric perfusion products creates a lucrative market for medical device manufacturers and suppliers. Investors can focus on importing and distributing high-quality oxygenators, blood pumps, and perfusion circuits. There is also potential for local manufacturing and assembly to reduce import dependency and lower costs. However, navigating the countrys regulatory requirements for medical devices remains a key challenge.
Government Policy of the market
Regulatory bodies such as the Food and Drug Administration Philippines (FDA Philippines) oversee the approval and distribution of pediatric perfusion products to maintain safety and quality standards. The governments push for medical device localization and procurement policies for government hospitals encourages the use of high-quality, cost-effective solutions. However, strict import regulations and lengthy approval processes may slow market growth, especially for new international products.
Key Highlights of the Report:
- Philippines Pediatric Perfusion Products Market Outlook
- Market Size of Philippines Pediatric Perfusion Products Market, 2024
- Forecast of Philippines Pediatric Perfusion Products Market, 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Revenues & Volume for the Period 2021- 2031
- Philippines Pediatric Perfusion Products Market Trend Evolution
- Philippines Pediatric Perfusion Products Market Drivers and Challenges
- Philippines Pediatric Perfusion Products Price Trends
- Philippines Pediatric Perfusion Products Porter's Five Forces
- Philippines Pediatric Perfusion Products Industry Life Cycle
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Pediatric Oxygenators for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Pediatric Arterial Filters for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Pediatric Cannulae for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Hemoconcentrators for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Reservoirs for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By End-user for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Specialty Clinics for the Period 2021- 2031
- Historical Data and Forecast of Philippines Pediatric Perfusion Products Market Revenues & Volume By Others for the Period 2021- 2031
- Philippines Pediatric Perfusion Products Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By End-user
- Philippines Pediatric Perfusion Products Top Companies Market Share
- Philippines Pediatric Perfusion Products Competitive Benchmarking By Technical and Operational Parameters
- Philippines Pediatric Perfusion Products Company Profiles
- Philippines Pediatric Perfusion Products Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Philippines Pediatric Perfusion Products Market Overview |
3.1 Philippines Country Macro Economic Indicators |
3.2 Philippines Pediatric Perfusion Products Market Revenues & Volume, 2021 & 2031F |
3.3 Philippines Pediatric Perfusion Products Market - Industry Life Cycle |
3.4 Philippines Pediatric Perfusion Products Market - Porter's Five Forces |
3.5 Philippines Pediatric Perfusion Products Market Revenues & Volume Share, By Product, 2021 & 2031F |
3.6 Philippines Pediatric Perfusion Products Market Revenues & Volume Share, By End-user, 2021 & 2031F |
4 Philippines Pediatric Perfusion Products Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of pediatric cardiovascular diseases in the Philippines |
4.2.2 Growing awareness about the importance of pediatric perfusion products for surgeries |
4.2.3 Technological advancements leading to the development of more efficient and safer products |
4.3 Market Restraints |
4.3.1 High cost associated with pediatric perfusion products impacting affordability |
4.3.2 Limited healthcare infrastructure and resources in certain regions of the Philippines |
4.3.3 Stringent regulatory requirements for pediatric medical devices |
5 Philippines Pediatric Perfusion Products Market Trends |
6 Philippines Pediatric Perfusion Products Market, By Types |
6.1 Philippines Pediatric Perfusion Products Market, By Product |
6.1.1 Overview and Analysis |
6.1.2 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Product, 2021- 2031F |
6.1.3 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Pediatric Oxygenators, 2021- 2031F |
6.1.4 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Pediatric Arterial Filters, 2021- 2031F |
6.1.5 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Pediatric Cannulae, 2021- 2031F |
6.1.6 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Hemoconcentrators, 2021- 2031F |
6.1.7 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Reservoirs, 2021- 2031F |
6.1.8 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Others, 2021- 2031F |
6.2 Philippines Pediatric Perfusion Products Market, By End-user |
6.2.1 Overview and Analysis |
6.2.2 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.2.3 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Specialty Clinics, 2021- 2031F |
6.2.4 Philippines Pediatric Perfusion Products Market Revenues & Volume, By Others, 2021- 2031F |
7 Philippines Pediatric Perfusion Products Market Import-Export Trade Statistics |
7.1 Philippines Pediatric Perfusion Products Market Export to Major Countries |
7.2 Philippines Pediatric Perfusion Products Market Imports from Major Countries |
8 Philippines Pediatric Perfusion Products Market Key Performance Indicators |
8.1 Adoption rate of pediatric perfusion products in major hospitals and healthcare facilities |
8.2 Number of pediatric cardiac surgeries performed using perfusion products |
8.3 Rate of complications or adverse events associated with pediatric perfusion products |
8.4 Level of investment in research and development for pediatric perfusion products |
8.5 Patient satisfaction and outcomes following the use of pediatric perfusion products |
9 Philippines Pediatric Perfusion Products Market - Opportunity Assessment |
9.1 Philippines Pediatric Perfusion Products Market Opportunity Assessment, By Product, 2021 & 2031F |
9.2 Philippines Pediatric Perfusion Products Market Opportunity Assessment, By End-user, 2021 & 2031F |
10 Philippines Pediatric Perfusion Products Market - Competitive Landscape |
10.1 Philippines Pediatric Perfusion Products Market Revenue Share, By Companies, 2024 |
10.2 Philippines Pediatric Perfusion Products Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















