Poland Ophthalmic Devices Market (2025-2031) Outlook | Industry, Value, Share, Size, Growth, Companies, Revenue, Analysis, Trends & Forecast
| Product Code: ETC367875 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Poland Ophthalmic Devices Market: Import Trend Analysis
In the Poland ophthalmic devices market, the import trend experienced a slight decline from 2023 to 2024, with a growth rate of -0.94%. However, the compound annual growth rate (CAGR) for imports from 2020 to 2024 stood at a healthy 9.45%. This dip in import momentum could be attributed to temporary shifts in demand patterns or adjustments in trade policies during that period.
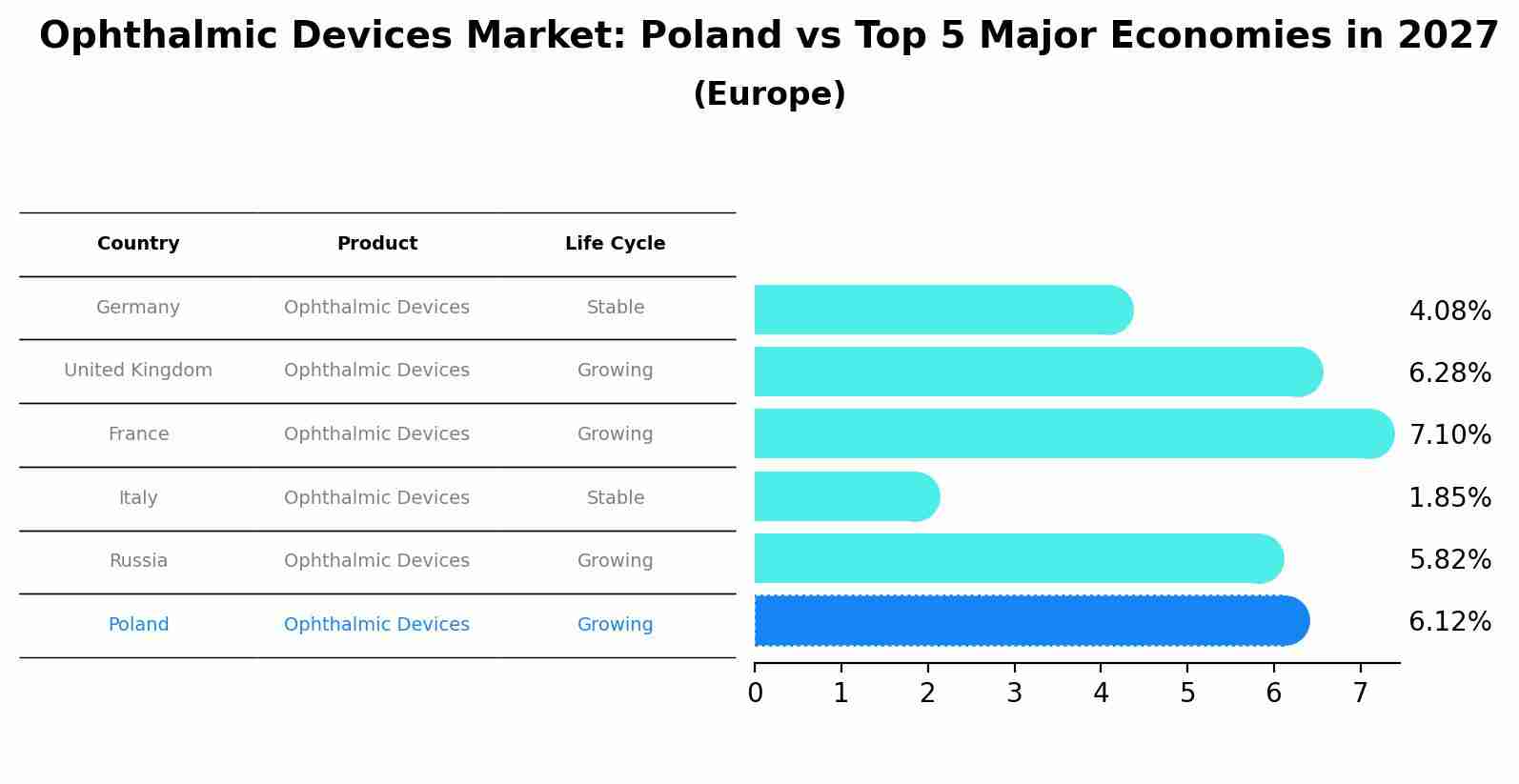
Ophthalmic Devices Market: Poland vs Top 5 Major Economies in 2027 (Europe)
In the Europe region, the Ophthalmic Devices market in Poland is projected to expand at a growing growth rate of 6.12% by 2027. The largest economy is Germany, followed by United Kingdom, France, Italy and Russia.
Poland Ophthalmic Devices Market Synopsis
The Poland Ophthalmic Devices Market is experiencing steady growth driven by factors such as the rising prevalence of eye diseases, an aging population, increasing awareness about eye health, and technological advancements in the field. The market includes various segments such as diagnostic and monitoring devices, surgical devices, vision care products, and others. Key players in the market are focusing on product innovation, strategic collaborations, and expansion of their distribution networks to gain a competitive edge. With a growing demand for advanced ophthalmic devices and increasing healthcare expenditure in Poland, the market is expected to continue expanding in the coming years. However, challenges such as stringent regulatory requirements and pricing pressure may impact market growth to some extent.
Poland Ophthalmic Devices Market Trends
The Poland Ophthalmic Devices Market is currently witnessing several key trends. One of the significant trends is the increasing adoption of advanced technologies such as femtosecond laser systems for cataract surgery and optical coherence tomography (OCT) for diagnostics. The market is also experiencing a growing demand for minimally invasive procedures and innovative surgical techniques, driving the development of new ophthalmic devices. Additionally, there is a rising prevalence of eye disorders and age-related macular degeneration in Poland, leading to a higher demand for ophthalmic devices for diagnosis and treatment. The market players are focusing on product advancements, strategic collaborations, and geographical expansions to capitalize on these trends and gain a competitive edge in the Poland Ophthalmic Devices Market.
Poland Ophthalmic Devices Market Challenges
In the Poland Ophthalmic Devices Market, some key challenges include increasing competition from both domestic and international players, pricing pressures due to a growing number of market entrants offering similar products, and regulatory hurdles related to product approvals and compliance. Additionally, the rapid technological advancements in the field of ophthalmology require companies to constantly innovate and upgrade their products to stay competitive. Market fragmentation and varying reimbursement policies across different regions within Poland also pose challenges for companies trying to establish a strong market presence. Overall, navigating these challenges requires a deep understanding of the market dynamics, strong regulatory knowledge, and strategic pricing and marketing strategies to differentiate products and succeed in the competitive landscape.
Poland Ophthalmic Devices Market Investment Opportunities
The Poland Ophthalmic Devices Market offers various investment opportunities due to the increasing prevalence of eye disorders, growing aging population, and advancements in healthcare infrastructure. The market for ophthalmic devices in Poland includes products such as diagnostic devices, surgical instruments, vision care products, and more. Investing in innovative technologies like laser devices for vision correction, advanced diagnostic equipment for early detection of eye diseases, and minimally invasive surgical tools can be lucrative. Additionally, there is a rising demand for contact lenses, glasses, and other vision care products among the Polish population. Collaborating with local healthcare providers, expanding distribution networks, and staying updated on regulatory changes can help investors capitalize on the expanding ophthalmic devices market in Poland.
Jordan Agar Market Government Policies
In Poland, the Ophthalmic Devices Market is regulated by the European Union`s Medical Devices Regulation (MDR) which sets requirements for the safety and performance of medical devices, including ophthalmic devices. The Polish government has implemented these regulations to ensure the quality, safety, and efficacy of ophthalmic devices in the market. Additionally, the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products (URPLWMiPB) oversees the registration and approval process for ophthalmic devices to be marketed in Poland. Companies operating in the Poland Ophthalmic Devices Market must comply with these regulations to ensure the availability of high-quality and safe products for patients in the country.
Poland Ophthalmic Devices Market Future Outlook
The Poland Ophthalmic Devices Market is expected to exhibit steady growth in the coming years, driven by factors such as the increasing prevalence of eye diseases, an aging population, and technological advancements in ophthalmic devices. The market is likely to expand due to rising awareness about eye health, growing adoption of minimally invasive surgical procedures, and the increasing demand for advanced diagnostic and monitoring devices. Additionally, government initiatives to improve healthcare infrastructure and access to eye care services are expected to contribute to market growth. However, challenges such as pricing pressures, regulatory hurdles, and competition from alternative treatment options may pose constraints on market development. Overall, the Poland Ophthalmic Devices Market is projected to show promising opportunities for market players in the near future.
Key Highlights of the Report:
- Poland Ophthalmic Devices Market Outlook
- Market Size of Poland Ophthalmic Devices Market, 2024
- Forecast of Poland Ophthalmic Devices Market, 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Revenues & Volume for the Period 2021 - 2031
- Poland Ophthalmic Devices Market Trend Evolution
- Poland Ophthalmic Devices Market Drivers and Challenges
- Poland Ophthalmic Devices Price Trends
- Poland Ophthalmic Devices Porter's Five Forces
- Poland Ophthalmic Devices Industry Life Cycle
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Product for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Optical Coherence Tomography Scanners for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Fundus Cameras for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Perimeters/Visual Field Analyzers for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Autorefractors and Keratometers for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Ophthalmic Ultrasound Imaging Systems for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Ophthalmic Pachymeters for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Tonometers for the Period 2021 - 2031
- Historical Data and Forecast of Poland Optical Coherence Tomography Scanners Ophthalmic Devices Market Revenues & Volume By Slit Lamps for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Cataract for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Vitreo retinal disorders for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Glaucoma for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Refractor Disorders for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By End-use for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Hospitals and Eye Clinics for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Academic and Research Laboratory for the Period 2021 - 2031
- Historical Data and Forecast of Poland Ophthalmic Devices Market Revenues & Volume By Others for the Period 2021 - 2031
- Poland Ophthalmic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End-use
- Poland Ophthalmic Devices Top Companies Market Share
- Poland Ophthalmic Devices Competitive Benchmarking By Technical and Operational Parameters
- Poland Ophthalmic Devices Company Profiles
- Poland Ophthalmic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















