United States (US) Carcinoembryonic Antigen Market (2025-2031) | Size & Revenue, Industry, Competitive Landscape, Segmentation, Analysis, Share, Outlook, Growth, Forecast, Trends, Companies, Value
| Product Code: ETC9961784 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
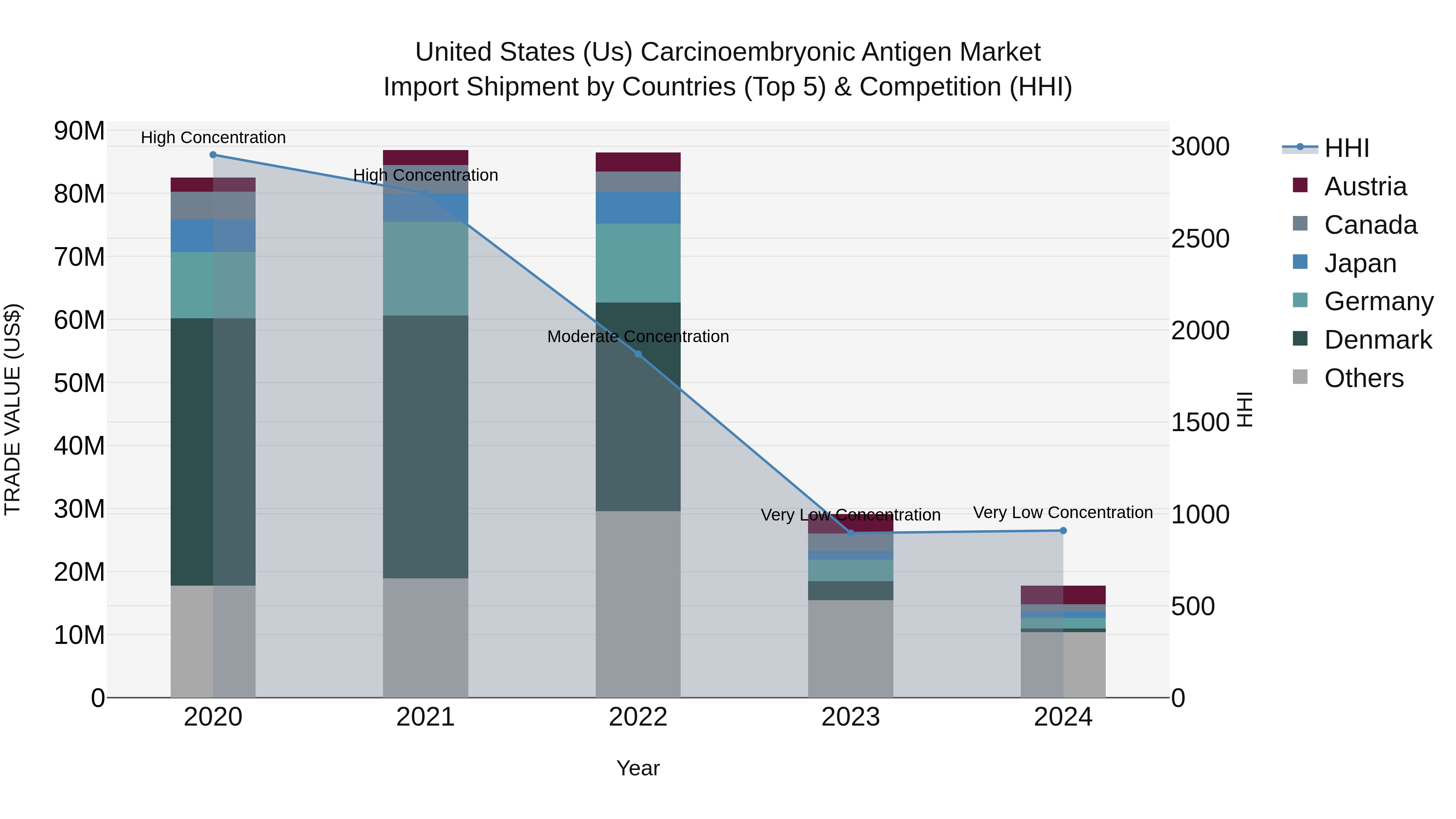
United States (US) Carcinoembryonic Antigen Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, the United States saw a significant decline in carcinoembryonic antigen import shipments, with a negative CAGR of -31.87% from 2020 to 2024. The top exporting countries to the USA were Austria, Metropolitan France, Italy, Germany, and Brazil. Despite the low concentration indicated by the Herfindahl-Hirschman Index (HHI), the growth rate plummeted by -39.06% from 2023 to 2024. This data suggests a challenging market environment for carcinoembryonic antigen imports into the United States in 2024.
Key Highlights of the Report:
- United States (US) Carcinoembryonic Antigen Market Outlook
- Market Size of United States (US) Carcinoembryonic Antigen Market, 2024
- Forecast of United States (US) Carcinoembryonic Antigen Market, 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Revenues & Volume for the Period 2021- 2031
- United States (US) Carcinoembryonic Antigen Market Trend Evolution
- United States (US) Carcinoembryonic Antigen Market Drivers and Challenges
- United States (US) Carcinoembryonic Antigen Price Trends
- United States (US) Carcinoembryonic Antigen Porter's Five Forces
- United States (US) Carcinoembryonic Antigen Industry Life Cycle
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Colorectal Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Pancreatic Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Ovarian Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Breast Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Thyroid Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Carcinoembryonic Antigen Market Revenues & Volume By Others for the Period 2021- 2031
- United States (US) Carcinoembryonic Antigen Import Export Trade Statistics
- Market Opportunity Assessment By Application
- United States (US) Carcinoembryonic Antigen Top Companies Market Share
- United States (US) Carcinoembryonic Antigen Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Carcinoembryonic Antigen Company Profiles
- United States (US) Carcinoembryonic Antigen Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) Carcinoembryonic Antigen Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) Carcinoembryonic Antigen Market - Industry Life Cycle |
3.4 United States (US) Carcinoembryonic Antigen Market - Porter's Five Forces |
3.5 United States (US) Carcinoembryonic Antigen Market Revenues & Volume Share, By Application, 2021 & 2031F |
4 United States (US) Carcinoembryonic Antigen Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of cancer in the US population |
4.2.2 Rising demand for early cancer detection and diagnosis |
4.2.3 Technological advancements in CEA testing methods |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for CEA testing |
4.3.2 High cost associated with CEA testing procedures |
4.3.3 Limited awareness about CEA testing among the general population |
5 United States (US) Carcinoembryonic Antigen Market Trends |
6 United States (US) Carcinoembryonic Antigen Market, By Types |
6.1 United States (US) Carcinoembryonic Antigen Market, By Application |
6.1.1 Overview and Analysis |
6.1.2 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Application, 2021- 2031F |
6.1.3 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Colorectal Cancer, 2021- 2031F |
6.1.4 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Pancreatic Cancer, 2021- 2031F |
6.1.5 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Ovarian Cancer, 2021- 2031F |
6.1.6 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Breast Cancer, 2021- 2031F |
6.1.7 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Thyroid Cancer, 2021- 2031F |
6.1.8 United States (US) Carcinoembryonic Antigen Market Revenues & Volume, By Others, 2021- 2031F |
7 United States (US) Carcinoembryonic Antigen Market Import-Export Trade Statistics |
7.1 United States (US) Carcinoembryonic Antigen Market Export to Major Countries |
7.2 United States (US) Carcinoembryonic Antigen Market Imports from Major Countries |
8 United States (US) Carcinoembryonic Antigen Market Key Performance Indicators |
8.1 Adoption rate of CEA testing among healthcare providers |
8.2 Number of research studies and clinical trials focusing on CEA testing |
8.3 Patient engagement and participation in CEA screening programs |
9 United States (US) Carcinoembryonic Antigen Market - Opportunity Assessment |
9.1 United States (US) Carcinoembryonic Antigen Market Opportunity Assessment, By Application, 2021 & 2031F |
10 United States (US) Carcinoembryonic Antigen Market - Competitive Landscape |
10.1 United States (US) Carcinoembryonic Antigen Market Revenue Share, By Companies, 2024 |
10.2 United States (US) Carcinoembryonic Antigen Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















