United States (US) Erythropoietin Market (2025-2031) | Industry, Share, Companies, Forecast, Trends, Growth, Outlook, Segmentation, Competitive Landscape, Size & Revenue, Value, Analysis
| Product Code: ETC9965413 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
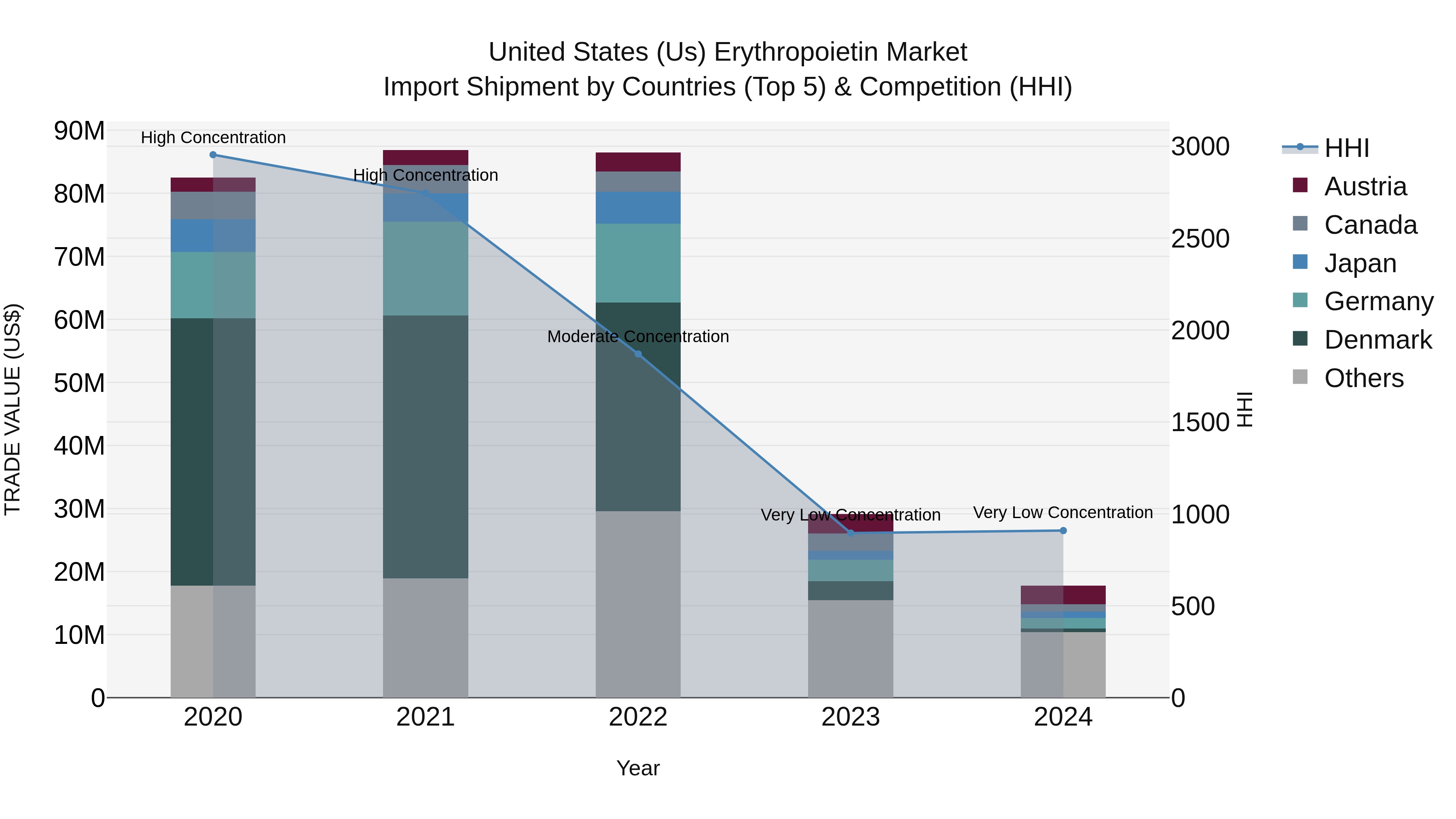
United States (US) Erythropoietin Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, the United States saw erythropoietin import shipments primarily coming from Austria, Metropolitan France, Italy, Germany, and Brazil. The market displayed low concentration levels with a Herfindahl-Hirschman Index (HHI) remaining very low. However, the compound annual growth rate (CAGR) from 2020 to 2024 was notably negative at -31.87%, with a further decline in the growth rate from 2023 to 2024 at -39.06%. These figures suggest a challenging market environment for erythropoietin imports into the US, potentially influenced by various factors impacting the industry during this period.
United States (US) Erythropoietin Market Overview
The United States Erythropoietin Market is a significant segment of the pharmaceutical industry, primarily driven by the increasing prevalence of chronic kidney disease and cancer-related anemia in the country. Erythropoietin, a hormone that stimulates red blood cell production, is widely used in the treatment of anemia associated with these conditions. The market is dominated by several key players that offer various forms of erythropoietin products, including recombinant human erythropoietin (rHuEPO) and biosimilars. Factors such as the growing aging population, advancements in healthcare infrastructure, and the rising demand for effective anemia management are expected to propel market growth. Regulatory approvals, product launches, and strategic collaborations are key strategies adopted by companies to maintain a competitive edge in the US Erythropoietin Market.
United States (US) Erythropoietin Market Trends and Opportunities
The US Erythropoietin market is experiencing growth due to increasing prevalence of chronic kidney disease, cancer, and anemia, driving the demand for erythropoietin stimulating agents (ESAs). The market is witnessing a shift towards biosimilar erythropoietin products, offering cost-effective alternatives to the originator products. Additionally, the expanding applications of erythropoietin in treating conditions such as chemotherapy-induced anemia and HIV-related anemia present new opportunities for market growth. The rise in healthcare expenditure, advancements in biotechnology, and increasing focus on personalized medicine are further propelling market expansion. Companies are focusing on strategic collaborations, product launches, and geographic expansions to capitalize on the growing demand for erythropoietin in the US market. Overall, the US Erythropoietin market is poised for significant growth and innovation in the coming years.
United States (US) Erythropoietin Market Challenges
The United States Erythropoietin Market faces several challenges, including increasing competition from biosimilar products, pricing pressure due to reimbursement cuts by payers, and regulatory scrutiny surrounding the prescribing and usage of erythropoietin-stimulating agents (ESAs). Additionally, the market is impacted by the shift towards value-based healthcare models, which emphasize cost-effectiveness and outcomes-based reimbursement. Another challenge is the potential for safety concerns related to the use of ESAs, such as cardiovascular risks in certain patient populations. To navigate these challenges, companies in the US Erythropoietin Market need to focus on innovation, differentiation, and strategic pricing strategies, while ensuring compliance with regulatory requirements and maintaining strong relationships with key stakeholders such as healthcare providers and payers.
United States (US) Erythropoietin Market Drivers
The United States Erythropoietin market is primarily driven by factors such as the increasing prevalence of chronic kidney disease and cancer, leading to a higher demand for erythropoietin stimulating agents (ESAs) to manage anemia. Additionally, the rising geriatric population in the US is also contributing to the growth of the market as elderly individuals are more prone to conditions requiring ESAs. Moreover, advancements in biotechnology and healthcare infrastructure are facilitating the development and adoption of innovative erythropoietin products, further propelling market expansion. The ongoing research and development activities focused on improving the efficacy and safety profiles of ESAs are expected to drive market growth in the coming years, as healthcare providers seek more effective treatment options for patients with anemia-related conditions.
United States (US) Erythropoietin Market Government Policies
In the United States, the Erythropoietin market is primarily regulated by the Food and Drug Administration (FDA) through the approval process for Erythropoietin-stimulating agents (ESAs). ESAs are used in the treatment of anemia associated with chronic kidney disease, chemotherapy, and other conditions. The FDA closely monitors the safety and efficacy of ESAs, requiring manufacturers to conduct post-market studies and provide updated labeling with risk information. Additionally, the Centers for Medicare & Medicaid Services (CMS) have implemented reimbursement policies for ESAs to control costs and promote appropriate use, particularly in the Medicare population. Overall, government policies aim to ensure patient safety, promote access to essential treatments, and control healthcare spending in the US Erythropoietin market.
United States (US) Erythropoietin Market Future Outlook
The future outlook for the United States Erythropoietin Market appears promising, driven by factors such as the increasing prevalence of chronic kidney diseases, cancer-related anemia, and other blood disorders that require erythropoietin therapy. Additionally, advancements in biotechnology and pharmaceutical research are likely to lead to the development of novel erythropoietin products with improved efficacy and safety profiles. The growing geriatric population and rising healthcare expenditures are expected to further propel market growth. However, challenges such as patent expirations, regulatory hurdles, and competition from biosimilars may hinder the market`s expansion. Overall, with the increasing demand for erythropoietin-based therapies and ongoing research and development activities, the US Erythropoietin Market is poised for steady growth in the coming years.
Key Highlights of the Report:
- United States (US) Erythropoietin Market Outlook
- Market Size of United States (US) Erythropoietin Market, 2024
- Forecast of United States (US) Erythropoietin Market, 2031
- Historical Data and Forecast of United States (US) Erythropoietin Revenues & Volume for the Period 2021- 2031
- United States (US) Erythropoietin Market Trend Evolution
- United States (US) Erythropoietin Market Drivers and Challenges
- United States (US) Erythropoietin Price Trends
- United States (US) Erythropoietin Porter's Five Forces
- United States (US) Erythropoietin Industry Life Cycle
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Drug Class for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Epoetin Alfa for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Epoetin Beta for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Darbepoetin Alfa for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Drug Type for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Biologics for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Biosimilar for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Drug Application for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Cancer for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Renal Disease for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Hematology for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Neurology for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Distribution Channel for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Hospital Pharmacy for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Retail Pharmacy for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Erythropoietin Market Revenues & Volume By Online Pharmacy for the Period 2021- 2031
- United States (US) Erythropoietin Import Export Trade Statistics
- Market Opportunity Assessment By Drug Class
- Market Opportunity Assessment By Drug Type
- Market Opportunity Assessment By Drug Application
- Market Opportunity Assessment By Distribution Channel
- United States (US) Erythropoietin Top Companies Market Share
- United States (US) Erythropoietin Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Erythropoietin Company Profiles
- United States (US) Erythropoietin Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) Erythropoietin Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) Erythropoietin Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) Erythropoietin Market - Industry Life Cycle |
3.4 United States (US) Erythropoietin Market - Porter's Five Forces |
3.5 United States (US) Erythropoietin Market Revenues & Volume Share, By Drug Class, 2021 & 2031F |
3.6 United States (US) Erythropoietin Market Revenues & Volume Share, By Drug Type, 2021 & 2031F |
3.7 United States (US) Erythropoietin Market Revenues & Volume Share, By Drug Application, 2021 & 2031F |
3.8 United States (US) Erythropoietin Market Revenues & Volume Share, By Distribution Channel, 2021 & 2031F |
4 United States (US) Erythropoietin Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of chronic kidney diseases requiring erythropoietin therapy |
4.2.2 Growing geriatric population in the United States |
4.2.3 Rising awareness about anemia management and treatment options |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for erythropoietin products |
4.3.2 High cost associated with erythropoietin therapy |
4.3.3 Competition from alternative treatment options such as iron supplements and blood transfusions |
5 United States (US) Erythropoietin Market Trends |
6 United States (US) Erythropoietin Market, By Types |
6.1 United States (US) Erythropoietin Market, By Drug Class |
6.1.1 Overview and Analysis |
6.1.2 United States (US) Erythropoietin Market Revenues & Volume, By Drug Class, 2021- 2031F |
6.1.3 United States (US) Erythropoietin Market Revenues & Volume, By Epoetin Alfa, 2021- 2031F |
6.1.4 United States (US) Erythropoietin Market Revenues & Volume, By Epoetin Beta, 2021- 2031F |
6.1.5 United States (US) Erythropoietin Market Revenues & Volume, By Darbepoetin Alfa, 2021- 2031F |
6.1.6 United States (US) Erythropoietin Market Revenues & Volume, By Others, 2021- 2031F |
6.2 United States (US) Erythropoietin Market, By Drug Type |
6.2.1 Overview and Analysis |
6.2.2 United States (US) Erythropoietin Market Revenues & Volume, By Biologics, 2021- 2031F |
6.2.3 United States (US) Erythropoietin Market Revenues & Volume, By Biosimilar, 2021- 2031F |
6.3 United States (US) Erythropoietin Market, By Drug Application |
6.3.1 Overview and Analysis |
6.3.2 United States (US) Erythropoietin Market Revenues & Volume, By Cancer, 2021- 2031F |
6.3.3 United States (US) Erythropoietin Market Revenues & Volume, By Renal Disease, 2021- 2031F |
6.3.4 United States (US) Erythropoietin Market Revenues & Volume, By Hematology, 2021- 2031F |
6.3.5 United States (US) Erythropoietin Market Revenues & Volume, By Neurology, 2021- 2031F |
6.3.6 United States (US) Erythropoietin Market Revenues & Volume, By Others, 2021- 2031F |
6.4 United States (US) Erythropoietin Market, By Distribution Channel |
6.4.1 Overview and Analysis |
6.4.2 United States (US) Erythropoietin Market Revenues & Volume, By Hospital Pharmacy, 2021- 2031F |
6.4.3 United States (US) Erythropoietin Market Revenues & Volume, By Retail Pharmacy, 2021- 2031F |
6.4.4 United States (US) Erythropoietin Market Revenues & Volume, By Online Pharmacy, 2021- 2031F |
7 United States (US) Erythropoietin Market Import-Export Trade Statistics |
7.1 United States (US) Erythropoietin Market Export to Major Countries |
7.2 United States (US) Erythropoietin Market Imports from Major Countries |
8 United States (US) Erythropoietin Market Key Performance Indicators |
8.1 Number of patients receiving erythropoietin therapy |
8.2 Average dosages of erythropoietin administered per patient |
8.3 Adoption rates of erythropoietin biosimilars |
8.4 Research and development investment in next-generation erythropoietin products |
9 United States (US) Erythropoietin Market - Opportunity Assessment |
9.1 United States (US) Erythropoietin Market Opportunity Assessment, By Drug Class, 2021 & 2031F |
9.2 United States (US) Erythropoietin Market Opportunity Assessment, By Drug Type, 2021 & 2031F |
9.3 United States (US) Erythropoietin Market Opportunity Assessment, By Drug Application, 2021 & 2031F |
9.4 United States (US) Erythropoietin Market Opportunity Assessment, By Distribution Channel, 2021 & 2031F |
10 United States (US) Erythropoietin Market - Competitive Landscape |
10.1 United States (US) Erythropoietin Market Revenue Share, By Companies, 2024 |
10.2 United States (US) Erythropoietin Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















