US Medical Device Adhesive Market (2025-2031) | Outlook, Companies, Size, Revenue, Industry, Value, Trends, Growth, Analysis, Forecast & Share
Market Forecast By Resin Type (Light Curing, Cyanoacrylates, Acrylic, Epoxy, Silicone, & Polyurethane), By Application (Needles, Catheters, Tube Sets, Masks, Polycarbonate Devices, Pacemaker) And Competitive Landscape
| Product Code: ETC4511402 | Publication Date: Jul 2023 | Updated Date: Nov 2025 | Product Type: Report | |
| Publisher: 6Wresearch | Author: Ravi Bhandari | No. of Pages: 85 | No. of Figures: 45 | No. of Tables: 25 |
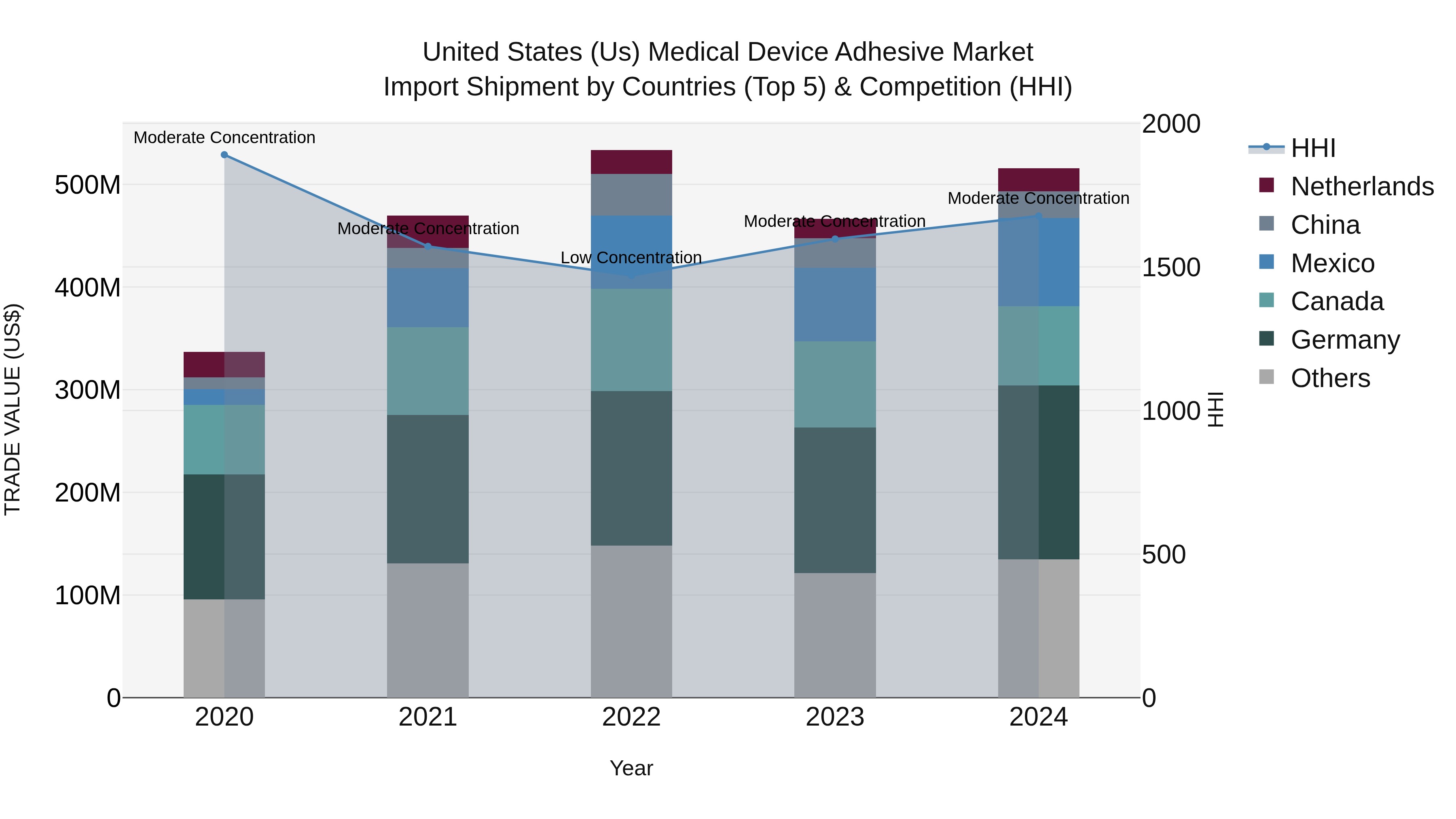
United States (US) Medical Device Adhesive Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The United States continues to see strong growth in medical device adhesive imports, with key exporting countries including Germany, Mexico, Canada, China, and the Netherlands. The industry shows a moderate concentration with a steadily increasing Compound Annual Growth Rate (CAGR) of 11.23% from 2020 to 2024. The growth rate in 2024 remains robust at 10.54%, indicating a positive trend in the market. These numbers reflect the ongoing demand for high-quality medical device adhesives in the US market and the importance of international trade relationships in meeting this demand.
US Medical Device Adhesive Market Highlights
| Report Name | US Medical Device Adhesive Market |
| Forecast period | 2025-2031 |
| CAGR | 10.4% |
| Growing Sector | Healthcare |
Topics Covered in the US Medical Device Adhesive Market Report
The US Medical Device Adhesive market report thoroughly covers the market by resin type, by application and competitive Landscape. The report provides an unbiased and detailed analysis of the on-going market trends, opportunities/high growth areas, and market drivers which would help the stakeholders to devise and align their market strategies according to the current and future market dynamics.
US Medical Device Adhesive Market Synopsis
The US medical device adhesive market is experiencing significant growth, driven by the increasing demand for medical devices that require strong, durable, and biocompatible adhesives. These adhesives are critical for ensuring the reliability and performance of medical devices in various applications, ranging from surgical instruments to wearable health monitors. Advances in adhesive technology have expanded the possibilities for medical device innovation, enabling the development of more complex and minimally invasive devices. This growth is further supported by stringent regulatory standards that demand high-quality and safe adhesives, reinforcing the importance of advanced adhesive solutions in the medical field. Additionally, several emerging trends are shaping the future of the US medical device adhesive market. The demand for wearable medical devices, for example, is soaring, necessitating adhesives that are gentle on the skin yet resilient enough to maintain device integrity under various conditions. Furthermore, the push for miniaturization of medical devices calls for adhesives that can bond small components reliably without compromising device performance. Another significant trend is the increasing preference for environmentally friendly and biodegradable adhesives, reflecting a broader industry shift towards sustainability. Lastly, advancements in adhesive technologies are enabling the development of smart adhesives that can provide additional functionalities, such as drug delivery or monitoring of device status, paving the way for innovative medical device applications.
According to 6Wresearch, US Medical Device Adhesive market size is projected to grow at a CAGR of 10.4% during 2025-2031. The growth of the US medical device adhesive market is propelled by several key factors. Firstly, the aging population in the US is increasing the demand for medical devices, including those for long-term and home-based care, which often rely on advanced adhesives for functionality and durability. Secondly, the technological advancements in medical devices, such as the integration of electronics for smart capabilities, necessitate the use of sophisticated adhesives that can withstand various operating conditions while ensuring patient safety. Another significant driver is the healthcare industry's continuous pursuit of reducing surgical times and improving patient recovery periods, which is steering the development of novel adhesive solutions for minimally invasive devices. Additionally, regulatory pressures and healthcare policies are pushing for higher standards of care and device reliability, further fueling the demand for high-quality medical device adhesives.
Government Initiatives Introduced in the US Medical Device Adhesive Market
Government initiatives play a crucial role in shaping the landscape of the medical device adhesive market. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) are implementing stricter guidelines to ensure the safety and efficacy of medical device adhesives used in various applications. These initiatives not only enforce compliance standards but also encourage innovation by setting benchmarks for adhesive performance, biocompatibility, and environmental sustainability. Furthermore, grants and funding opportunities provided by government agencies for research and development in biodegradable and non-toxic adhesive technologies propel advancements in this field. Steadily, these agendas have boosted the US Medical Device Adhesive Market Share. Additionally, such support underscores the government's commitment to fostering healthcare innovation while prioritizing patient safety and environmental stewardship.
Key Players in the US Medical Device Adhesive Market
Key players in the medical device adhesives sector include Henkel AG & Co. KGaA, 3M Company, Dow Chemical Company, and Cyberbond LLC. Henkel leads with its innovative adhesive technologies that provide durability and safety across various medical applications, while 3M is renowned for its wide range of biocompatible adhesive solutions catering to healthcare needs. Dow Chemical Company contributes with its specialized polymers that enhance the performance and longevity of medical adhesives. Cyberbond LLC, on the other hand, stands out for its rapid-cure adhesives that are ideal for high-volume production needs. Moreover, the businesses’ grasp massive US Medical Device Adhesive Market Revenues. Further, these companies not only drive technological advancements in the field but also adhere to stringent regulatory standards, ensuring their products meet the highest levels of safety and efficacy.
Future Insights of the US Medical Device Adhesive Market
The future of medical device adhesives looks promising with the anticipated integration of nanotechnology and bioengineering. These advancements are expected to yield adhesives with superior bond strength, biodegradability, and enhanced healing properties. Researchers are exploring the use of nanomaterials to create adhesives that can precisely mimic the natural adhesion properties found in biological systems, such as the gecko's footpad or mussel's underwater adhesive abilities. This biomimicry approach promises not only to revolutionize the adhesive qualities but also to introduce adhesives that are more compatible with the human body, reducing the risk of adverse reactions. Additionally, the ongoing push for sustainability and environmental responsibility is likely to drive the development of green adhesive technologies that are non-toxic, made from renewable resources, and fully biodegradable. Together, these innovations hold the potential to significantly impact the medical device sector, offering solutions that enhance patient care while adhering to environmental and safety standards.
Market Analysis by Resin Type
According to Ravi Bhandari, Research Head, 6Wresearch, in the realm of medical adhesive technologies, several types of resins play crucial roles due to their distinct properties and applications. Light Curing resins are favored for their rapid curing time under exposure to light, making them suitable for delicate medical equipment assembly. Cyanoacrylates, known for their strong and quick bonding capabilities, are often used in disposable medical devices. Acrylic resins provide a balance of strength and flexibility, critical for wearables and devices requiring prolonged skin contact. Epoxy resins are valued for their high resistance to chemicals and temperature, crucial for sterilizable medical instruments. Lastly, Silicone and Polyurethane resins are preferred for their biocompatibility and flexibility, making them ideal for creating comfortable, yet durable, medical adhesives and coatings. Each resin type offers unique advantages, catering to the diverse needs of medical device manufacturing and patient care.
Market Analysis by Application
The application of these advanced adhesives extends to a wide variety of medical devices, enhancing their functionality and safety. For instance, Needles benefit from strong, durable bonds that prevent leakage and ensure precise medication delivery. Catheters utilize biocompatible adhesives to minimize patient discomfort and reduce the risk of infection. Tube Sets, essential for fluid delivery and ventilation systems, rely on adhesives that form secure, leak-proof connections. Masks, including those used in surgery and respiratory therapy, need adhesives that can withstand moisture and provide a comfortable fit against the skin. Polycarbonate Devices, known for their clarity and strength, use specific resins that bond without compromising transparency or integrity. Lastly, adhesives in Pacemakers must be biocompatible, reliable, and capable of enduring the body's harsh internal environment. Each application underscores the critical role of adhesive technologies in modern medical care, ensuring devices perform as intended for patient well-being.
Key Attractiveness of the Report
- 10 Years Market Numbers.
- Historical Data Starting from 2021 to 2024.
- Base Year: 2024.
- Forecast Data until 2031.
- Key Performance Indicators Impacting the Market.
- Major Upcoming Developments and Projects.
Key Highlights of the Report:
- US Medical Device Adhesive Market Overview
- US Medical Device Adhesive Market Outlook
- US Medical Device Adhesive Market Forecast
- Market Size of United States (US) Medical Device Adhesive Market, 2024
- Forecast of United States (US) Medical Device Adhesive Market, 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Revenues & Volume for the Period 2021 - 2031
- United States (US) Medical Device Adhesive Market Trend Evolution
- United States (US) Medical Device Adhesive Market Drivers and Challenges
- United States (US) Medical Device Adhesive Price Trends
- United States (US) Medical Device Adhesive Porter's Five Forces
- United States (US) Medical Device Adhesive Industry Life Cycle
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Resin Type for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Light Curing for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Cyanoacrylates for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Acrylic for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Epoxy for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Silicone for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By & Polyurethane for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Needles for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Catheters for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Tube Sets for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume By Masks for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume, By Polycarbonate Devices for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Medical Device Adhesive Market Revenues & Volume, By Pacemaker for the Period 2021 - 2031
- United States (US) Medical Device Adhesive Import Export Trade Statistics
- Market Opportunity Assessment, By Resin Type
- Market Opportunity Assessment, By Application
- United States (US) Medical Device Adhesive Top Companies Market Share
- United States (US) Medical Device Adhesive Competitive Benchmarking, By Technical and Operational Parameters
- United States (US) Medical Device Adhesive Company Profiles
- United States (US) Medical Device Adhesive Key Strategic Recommendations
Markets Covered
The US Medical Device Adhesive market report provides a detailed analysis of the following market segments:
By Resin Type
- Light Curing
- Cyanoacrylates
- Acrylic
- Epoxy
- Silicone & Polyurethane
By Application
- Needles
- Catheters
- Tube Sets
- Masks
- Polycarbonate Devices
- Pacemaker
US Medical Device Adhesive Market (2025-2031): FAQs
| 1 Executive Summary |
| 2 Introduction |
| 2.1 Key Highlights of the Report |
| 2.2 Report Description |
| 2.3 Market Scope & Segmentation |
| 2.4 Research Methodology |
| 2.5 Assumptions |
| 3 United States (US) Medical Device Adhesive Market Overview |
| 3.1 United States (US) Country Macro Economic Indicators |
| 3.2 United States (US) Medical Device Adhesive Market Revenues & Volume, 2021 & 2031F |
| 3.3 United States (US) Medical Device Adhesive Market - Industry Life Cycle |
| 3.4 United States (US) Medical Device Adhesive Market - Porter's Five Forces |
| 3.5 United States (US) Medical Device Adhesive Market Revenues & Volume Share, By Resin Type, 2021 & 2031F |
| 3.6 United States (US) Medical Device Adhesive Market Revenues & Volume Share, By Application, 2021 & 2031F |
| 4 United States (US) Medical Device Adhesive Market Dynamics |
| 4.1 Impact Analysis |
| 4.2 Market Drivers |
| 4.2.1 Increasing demand for minimally invasive surgeries |
| 4.2.2 Technological advancements in medical device adhesives |
| 4.2.3 Rising prevalence of chronic diseases requiring medical devices |
| 4.2.4 Growing geriatric population in the United States |
| 4.3 Market Restraints |
| 4.3.1 Stringent regulatory requirements for medical device adhesives |
| 4.3.2 High cost associated with advanced medical device adhesives |
| 4.3.3 Limited reimbursement policies for medical device adhesives |
| 4.3.4 Competition from alternative wound closure methods |
| 5 United States (US) Medical Device Adhesive Market Trends |
| 6 United States (US) Medical Device Adhesive Market, By Types |
| 6.1 United States (US) Medical Device Adhesive Market, By Resin Type |
| 6.1.1 Overview and Analysis |
| 6.1.2 United States (US) Medical Device Adhesive Market Revenues & Volume, By Resin Type, 2021 - 2031F |
| 6.1.3 United States (US) Medical Device Adhesive Market Revenues & Volume, By Light Curing, 2021 - 2031F |
| 6.1.4 United States (US) Medical Device Adhesive Market Revenues & Volume, By Cyanoacrylates, 2021 - 2031F |
| 6.1.5 United States (US) Medical Device Adhesive Market Revenues & Volume, By Acrylic, 2021 - 2031F |
| 6.1.6 United States (US) Medical Device Adhesive Market Revenues & Volume, By Epoxy, 2021 - 2031F |
| 6.1.7 United States (US) Medical Device Adhesive Market Revenues & Volume, By Silicone, 2021 - 2031F |
| 6.1.8 United States (US) Medical Device Adhesive Market Revenues & Volume, By & Polyurethane, 2021 - 2031F |
| 6.2 United States (US) Medical Device Adhesive Market, By Application |
| 6.2.1 Overview and Analysis |
| 6.2.2 United States (US) Medical Device Adhesive Market Revenues & Volume, By Needles, 2021 - 2031F |
| 6.2.3 United States (US) Medical Device Adhesive Market Revenues & Volume, By Catheters, 2021 - 2031F |
| 6.2.4 United States (US) Medical Device Adhesive Market Revenues & Volume, By Tube Sets, 2021 - 2031F |
| 6.2.5 United States (US) Medical Device Adhesive Market Revenues & Volume, By Masks, 2021 - 2031F |
| 6.2.6 United States (US) Medical Device Adhesive Market Revenues & Volume, By Polycarbonate Devices, 2021 - 2031F |
| 6.2.7 United States (US) Medical Device Adhesive Market Revenues & Volume, By Pacemaker, 2021 - 2031F |
| 7 United States (US) Medical Device Adhesive Market Import-Export Trade Statistics |
| 7.1 United States (US) Medical Device Adhesive Market Export to Major Countries |
| 7.2 United States (US) Medical Device Adhesive Market Imports from Major Countries |
| 8 United States (US) Medical Device Adhesive Market Key Performance Indicators |
| 8.1 Adoption rate of new medical device adhesive technologies |
| 8.2 Number of FDA approvals for medical device adhesives |
| 8.3 Rate of growth in the number of medical procedures utilizing adhesives |
| 9 United States (US) Medical Device Adhesive Market - Opportunity Assessment |
| 9.1 United States (US) Medical Device Adhesive Market Opportunity Assessment, By Resin Type, 2021 & 2031F |
| 9.2 United States (US) Medical Device Adhesive Market Opportunity Assessment, By Application, 2021 & 2031F |
| 10 United States (US) Medical Device Adhesive Market - Competitive Landscape |
| 10.1 United States (US) Medical Device Adhesive Market Revenue Share, By Companies, 2024 |
| 10.2 United States (US) Medical Device Adhesive Market Competitive Benchmarking, By Operating and Technical Parameters |
| 11 Company Profiles |
| 12 Recommendations |
| 13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















