United States (US) PTA Balloon Catheter Market (2025-2031) | Industry, Value, Companies, Share, Competitive Landscape, Size & Revenue, Trends, Outlook, Growth, Segmentation, Analysis, Forecast
| Product Code: ETC9972676 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Sumit Sagar | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
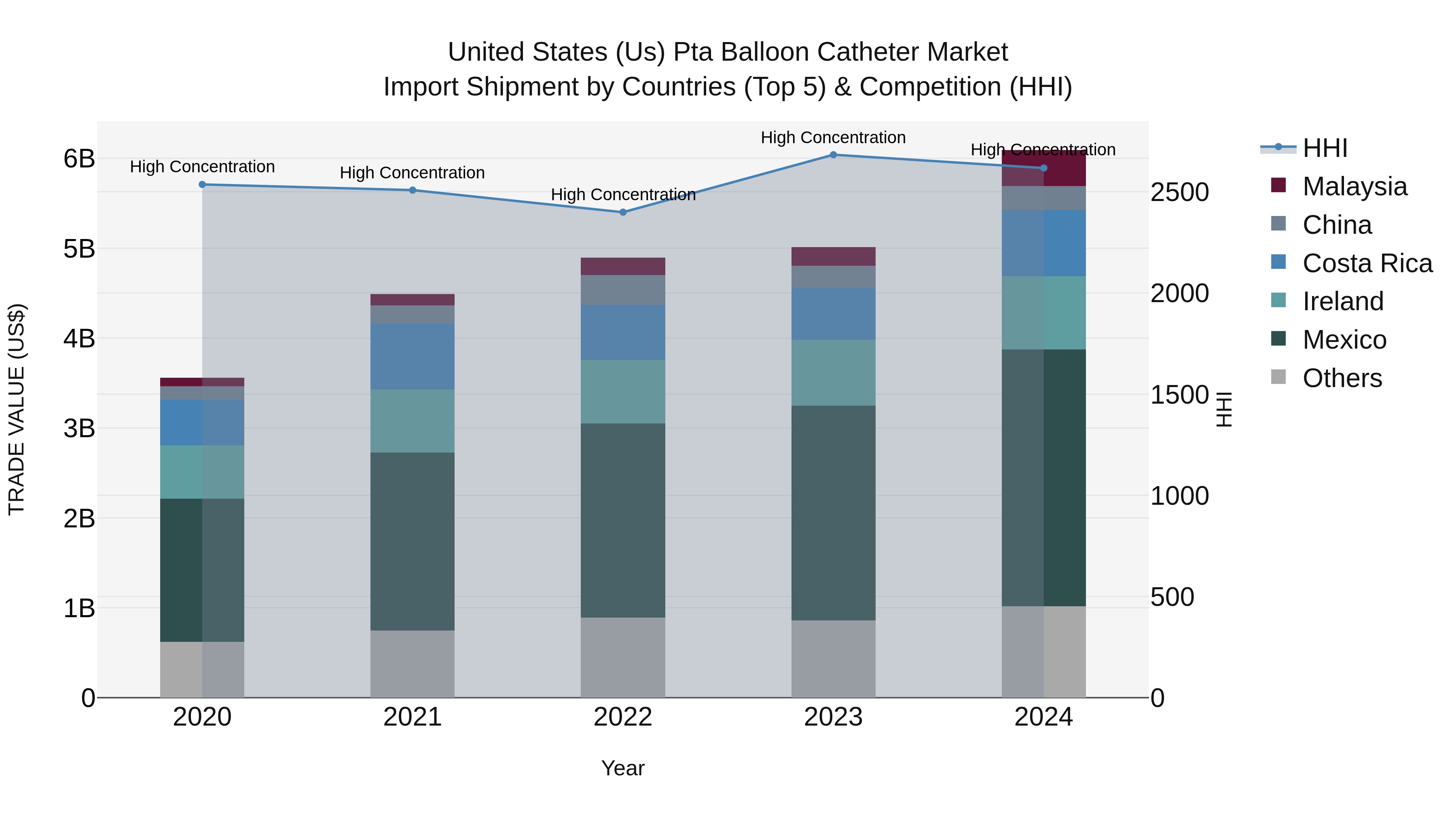
United States (US) Pta Balloon Catheter Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The United States continues to be a key importer of PTA balloon catheters, with Mexico, Ireland, Costa Rica, Malaysia, and China emerging as top exporting countries in 2024. The high Herfindahl-Hirschman Index (HHI) indicates a concentrated market, while the impressive Compound Annual Growth Rate (CAGR) of 14.38% from 2020 to 2024 reflects sustained growth. Moreover, the notable growth rate of 21.5% from 2023 to 2024 suggests a strong momentum in import shipments, underscoring the significance of these countries in meeting the demand for PTA balloon catheters in the US market.
United States (US) PTA Balloon Catheter Market Overview
The United States PTA Balloon Catheter Market is a growing segment within the medical device industry, driven by the increasing prevalence of peripheral artery disease (PAD) and demand for minimally invasive treatment options. PTA balloon catheters are widely used in the treatment of PAD as they effectively dilate narrowed or blocked arteries, improving blood flow and reducing symptoms such as leg pain and cramping. Key players in the US market include major medical device companies such as Medtronic, Boston Scientific, and Abbott Laboratories, who offer a variety of PTA balloon catheters with different sizes and features to cater to the diverse needs of healthcare providers and patients. The market is characterized by ongoing technological advancements, such as drug-coated balloon catheters, which are expected to further drive market growth in the coming years.
United States (US) PTA Balloon Catheter Market Trends and Opportunities
The US PTA Balloon Catheter market is currently experiencing a shift towards the adoption of advanced technologies such as drug-coated balloons and cutting-edge materials to improve patient outcomes. With the rising prevalence of cardiovascular diseases and the increasing geriatric population, there is a growing demand for minimally invasive procedures like percutaneous transluminal angioplasty (PTA), driving the market growth. Additionally, the focus on healthcare cost reduction and efficient treatment options is creating opportunities for innovative product development and strategic partnerships among key market players. The market is expected to witness continued expansion as healthcare providers seek more effective solutions for peripheral artery disease and other vascular conditions, leading to a favorable outlook for the US PTA Balloon Catheter market in the coming years.
United States (US) PTA Balloon Catheter Market Challenges
In the United States PTA balloon catheter market, some of the key challenges include intense competition among market players leading to pricing pressure, stringent regulatory requirements for product approval and market entry, reimbursement issues with insurance providers affecting product adoption rates, and the constant need for technological advancements to stay ahead in the market. Additionally, the market faces challenges related to the limited availability of skilled healthcare professionals trained in using PTA balloon catheters effectively, as well as the need for continuous investments in research and development to improve product efficacy and safety. Market dynamics such as changing healthcare policies, evolving patient preferences, and increasing healthcare costs also contribute to the challenges faced by companies operating in the US PTA balloon catheter market.
United States (US) PTA Balloon Catheter Market Drivers
The drivers fueling the growth of the US PTA balloon catheter market include the increasing prevalence of cardiovascular diseases and related risk factors such as obesity and diabetes, which are driving the demand for minimally invasive treatment options. Technological advancements in PTA balloon catheters, leading to improved efficacy and safety in treating peripheral artery disease, are also contributing to market growth. Additionally, the rising geriatric population in the US, who are more prone to vascular disorders, is driving the need for PTA balloon catheter procedures. Moreover, the growing awareness among healthcare professionals and patients about the benefits of minimally invasive procedures over traditional surgical interventions is further propelling the market expansion. Overall, these factors are expected to continue driving the growth of the US PTA balloon catheter market in the coming years.
United States (US) PTA Balloon Catheter Market Government Policies
The US PTA Balloon Catheter Market is subject to government policies that regulate medical devices, ensuring safety and efficacy. The Food and Drug Administration (FDA) oversees the approval process for PTA balloon catheters, requiring manufacturers to demonstrate the device`s safety and effectiveness before it can be marketed. Additionally, reimbursement policies set by government healthcare programs, such as Medicare, impact the market by influencing healthcare providers` decisions on device utilization. Changes in healthcare legislation and regulatory frameworks can also affect market dynamics, driving innovation and shaping competition among manufacturers in the US PTA Balloon Catheter Market. Overall, government policies play a crucial role in shaping the landscape of the US PTA Balloon Catheter Market by ensuring patient safety, promoting innovation, and influencing market access and utilization.
United States (US) PTA Balloon Catheter Market Future Outlook
The United States PTA balloon catheter market is expected to witness steady growth in the coming years due to the increasing prevalence of peripheral artery disease (PAD) and the rising geriatric population. Technological advancements in catheter design and materials, along with the growing adoption of minimally invasive procedures, are driving market expansion. Additionally, the rising awareness about the benefits of PTA balloon catheters in treating arterial blockages and improving patient outcomes will further fuel market growth. The competitive landscape is characterized by key players focusing on product innovation and strategic collaborations to gain a competitive edge. Overall, the US PTA balloon catheter market is poised for sustained growth in the foreseeable future as healthcare providers continue to prioritize effective and minimally invasive treatment options for vascular diseases.
Key Highlights of the Report:
- United States (US) PTA Balloon Catheter Market Outlook
- Market Size of United States (US) PTA Balloon Catheter Market, 2024
- Forecast of United States (US) PTA Balloon Catheter Market, 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Revenues & Volume for the Period 2021- 2031
- United States (US) PTA Balloon Catheter Market Trend Evolution
- United States (US) PTA Balloon Catheter Market Drivers and Challenges
- United States (US) PTA Balloon Catheter Price Trends
- United States (US) PTA Balloon Catheter Porter's Five Forces
- United States (US) PTA Balloon Catheter Industry Life Cycle
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Material Type for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Polyurethane for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Nylon for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Peripheral Artery Disease for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Coronary Artery Disease for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By End-use for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Hospital for the Period 2021- 2031
- Historical Data and Forecast of United States (US) PTA Balloon Catheter Market Revenues & Volume By Ambulatory Surgical Centers for the Period 2021- 2031
- United States (US) PTA Balloon Catheter Import Export Trade Statistics
- Market Opportunity Assessment By Material Type
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End-use
- United States (US) PTA Balloon Catheter Top Companies Market Share
- United States (US) PTA Balloon Catheter Competitive Benchmarking By Technical and Operational Parameters
- United States (US) PTA Balloon Catheter Company Profiles
- United States (US) PTA Balloon Catheter Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) PTA Balloon Catheter Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) PTA Balloon Catheter Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) PTA Balloon Catheter Market - Industry Life Cycle |
3.4 United States (US) PTA Balloon Catheter Market - Porter's Five Forces |
3.5 United States (US) PTA Balloon Catheter Market Revenues & Volume Share, By Material Type, 2021 & 2031F |
3.6 United States (US) PTA Balloon Catheter Market Revenues & Volume Share, By Application, 2021 & 2031F |
3.7 United States (US) PTA Balloon Catheter Market Revenues & Volume Share, By End-use, 2021 & 2031F |
4 United States (US) PTA Balloon Catheter Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of cardiovascular diseases in the US |
4.2.2 Technological advancements in PTA balloon catheter design and materials |
4.2.3 Growing adoption of minimally invasive procedures in healthcare |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for medical devices in the US |
4.3.2 High cost associated with PTA balloon catheter procedures |
4.3.3 Limited reimbursement policies for certain patient populations |
5 United States (US) PTA Balloon Catheter Market Trends |
6 United States (US) PTA Balloon Catheter Market, By Types |
6.1 United States (US) PTA Balloon Catheter Market, By Material Type |
6.1.1 Overview and Analysis |
6.1.2 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Material Type, 2021- 2031F |
6.1.3 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Polyurethane, 2021- 2031F |
6.1.4 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Nylon, 2021- 2031F |
6.2 United States (US) PTA Balloon Catheter Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Peripheral Artery Disease, 2021- 2031F |
6.2.3 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Coronary Artery Disease, 2021- 2031F |
6.3 United States (US) PTA Balloon Catheter Market, By End-use |
6.3.1 Overview and Analysis |
6.3.2 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Hospital, 2021- 2031F |
6.3.3 United States (US) PTA Balloon Catheter Market Revenues & Volume, By Ambulatory Surgical Centers, 2021- 2031F |
7 United States (US) PTA Balloon Catheter Market Import-Export Trade Statistics |
7.1 United States (US) PTA Balloon Catheter Market Export to Major Countries |
7.2 United States (US) PTA Balloon Catheter Market Imports from Major Countries |
8 United States (US) PTA Balloon Catheter Market Key Performance Indicators |
8.1 Average procedure time for PTA balloon catheter interventions |
8.2 Number of hospitals offering PTA balloon catheter procedures |
8.3 Adoption rate of advanced PTA balloon catheter technologies |
8.4 Patient satisfaction scores related to PTA balloon catheter procedures |
8.5 Percentage of complications or adverse events reported post-PTA balloon catheter interventions |
9 United States (US) PTA Balloon Catheter Market - Opportunity Assessment |
9.1 United States (US) PTA Balloon Catheter Market Opportunity Assessment, By Material Type, 2021 & 2031F |
9.2 United States (US) PTA Balloon Catheter Market Opportunity Assessment, By Application, 2021 & 2031F |
9.3 United States (US) PTA Balloon Catheter Market Opportunity Assessment, By End-use, 2021 & 2031F |
10 United States (US) PTA Balloon Catheter Market - Competitive Landscape |
10.1 United States (US) PTA Balloon Catheter Market Revenue Share, By Companies, 2024 |
10.2 United States (US) PTA Balloon Catheter Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















