Czech Republic Blood Transfusion Devices Market (2025-2031) | Revenue, Outlook, Forecast, Value, Companies, Growth, Trends, Analysis, Size, Industry & Share
| Product Code: ETC050996 | Publication Date: Jan 2021 | Updated Date: Sep 2025 | Product Type: Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 70 | No. of Figures: 35 | No. of Tables: 5 |
Blood Transfusion Devices Market: Czech Republic vs Top 5 Major Economies in 2027 (Europe)
By 2027, Czech Republic's Blood Transfusion Devices market is forecasted to achieve a negative growth rate of -0.00%, with Germany leading the Europe region, followed by United Kingdom, France, Italy and Russia.
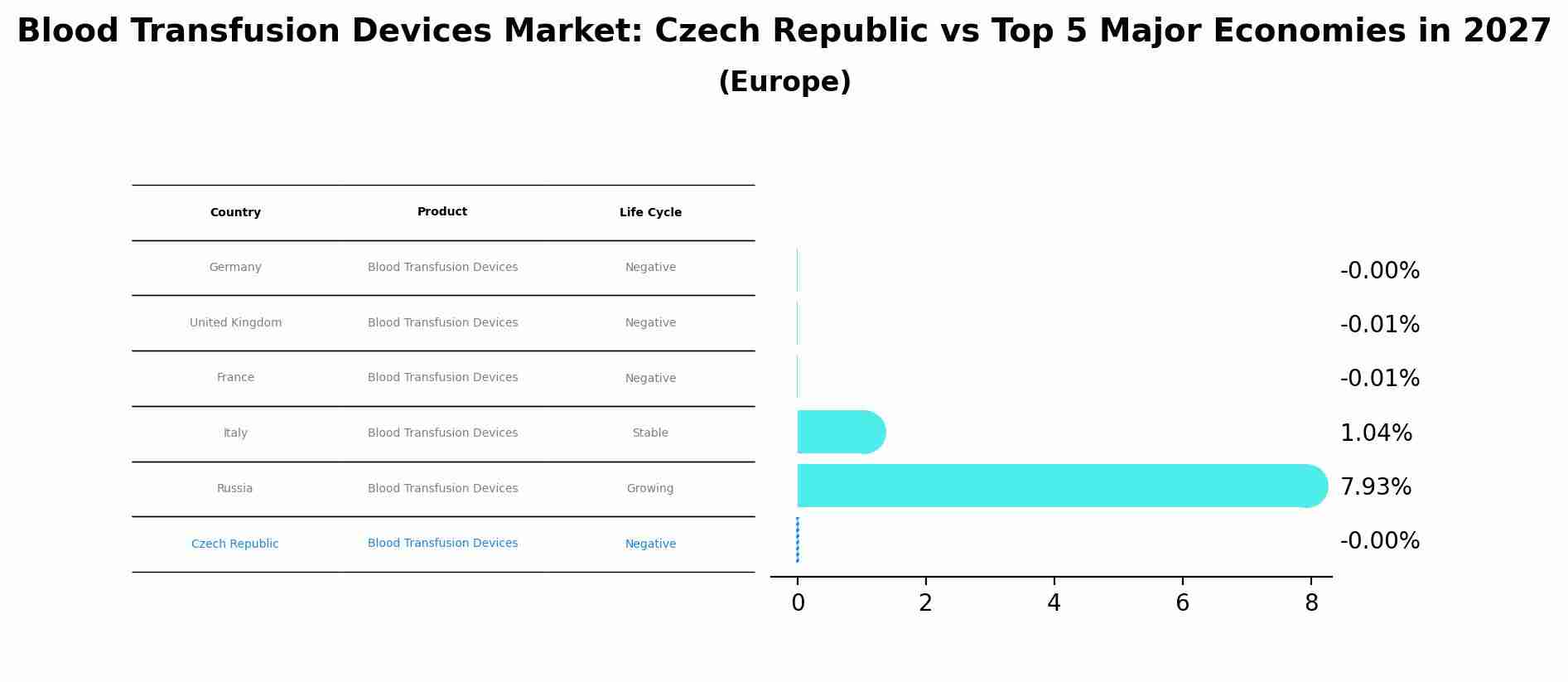
Czech Republic Blood Transfusion Devices Market Overview
The Czech Republic Blood Transfusion Devices Market is experiencing steady growth driven by increasing demand for blood transfusions in healthcare facilities. The market is characterized by the presence of key players offering a wide range of products such as blood bags, transfusion sets, infusion pumps, and needles. Technological advancements in blood transfusion devices, coupled with the rising number of patients requiring blood transfusions for various medical conditions, are further fueling market growth. Additionally, government initiatives to improve healthcare infrastructure and ensure the availability of safe blood transfusion devices are contributing to the market expansion. However, pricing pressures and regulatory challenges related to the quality control of blood transfusion devices present potential barriers to market growth in the Czech Republic.
Czech Republic Blood Transfusion Devices Market Trends
The Czech Republic Blood Transfusion Devices Market is witnessing several key trends. One significant trend is the increasing adoption of automated blood processing systems to improve efficiency and reduce the risk of human error in blood transfusion procedures. Another notable trend is the growing emphasis on safety measures, with a focus on the development of advanced blood filters and screening technologies to minimize the risk of transfusion-transmitted infections. Additionally, there is a rising demand for portable and compact blood transfusion devices to enhance mobility and flexibility in healthcare settings. Moreover, the market is seeing a shift towards the use of disposable blood transfusion sets and devices to ensure sterility and prevent cross-contamination. Overall, these trends indicate a growing focus on enhancing the safety, efficiency, and convenience of blood transfusion processes in the Czech Republic.
Czech Republic Blood Transfusion Devices Market Challenges
In the Czech Republic Blood Transfusion Devices Market, one of the main challenges is ensuring a stable supply of high-quality blood transfusion devices while dealing with budget constraints within the healthcare system. Additionally, the market may face regulatory hurdles in terms of compliance with stringent safety and quality standards for blood transfusion products. Another challenge is the need for continuous innovation and technological advancements in blood transfusion devices to enhance efficiency and patient safety. Moreover, competition from established international players and the need for effective distribution channels to reach healthcare facilities in remote areas could also pose challenges in this market. Overall, navigating these challenges requires strategic planning, strong partnerships with healthcare providers, and a deep understanding of the regulatory landscape in the Czech Republic.
Czech Republic Blood Transfusion Devices Market Investment Opportunities
The Czech Republic Blood Transfusion Devices Market presents promising investment opportunities due to the increasing demand for blood transfusion services in the country. With a growing number of surgeries, trauma cases, and chronic diseases requiring blood transfusions, the market for blood transfusion devices such as blood bags, filters, and transfusion sets is expected to expand. Additionally, advancements in technology and healthcare infrastructure in the Czech Republic are driving the adoption of innovative blood transfusion devices, creating a conducive environment for investment. Investors can capitalize on this market by exploring partnerships with local healthcare facilities, distributors, or manufacturers of blood transfusion devices to tap into the growing demand and contribute to improving healthcare services in the country.
Czech Republic Blood Transfusion Devices Market Government Policy
The Czech Republic Blood Transfusion Devices Market is governed by various regulations and policies aimed at ensuring the safety and quality of blood transfusion products. The market is regulated by the State Institute for Drug Control (SUKL), which oversees the approval and registration of blood transfusion devices. The Czech Republic follows the European Union regulations for blood transfusion devices to maintain high standards of safety and efficacy. Manufacturers must comply with Good Manufacturing Practice (GMP) guidelines and undergo rigorous testing and inspections to ensure product quality. Additionally, there are strict guidelines for blood donation and screening procedures to prevent the transmission of diseases through blood transfusions. Overall, the government policies in the Czech Republic emphasize the importance of patient safety and the reliability of blood transfusion devices in the market.
Czech Republic Blood Transfusion Devices Market Future Outlook
The future outlook for the Czech Republic Blood Transfusion Devices Market appears positive, with an increasing demand for blood transfusion services due to a growing prevalence of chronic diseases and an aging population. Technological advancements in blood transfusion devices, such as improved safety features and automation, are expected to drive market growth. Moreover, rising awareness about the importance of blood donation and government initiatives to enhance healthcare infrastructure are likely to further boost the market. However, pricing pressures and stringent regulatory requirements may pose challenges for market players. Overall, the Czech Republic Blood Transfusion Devices Market is anticipated to witness steady growth in the coming years, presenting opportunities for companies to innovate and expand their market presence.
Key Highlights of the Report:
- Czech Republic Blood Transfusion Devices Market Outlook
- Market Size of Czech Republic Blood Transfusion Devices Market, 2024
- Forecast of Czech Republic Blood Transfusion Devices Market, 2026
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Revenues & Volume for the Period 2021 - 2031
- Czech Republic Blood Transfusion Devices Market Trend Evolution
- Czech Republic Blood Transfusion Devices Market Drivers and Challenges
- Czech Republic Blood Transfusion Devices Price Trends
- Czech Republic Blood Transfusion Devices Porter's Five Forces
- Czech Republic Blood Transfusion Devices Industry Life Cycle
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Product for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Blood Bag and Accessory for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Blood Mixer for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Filter for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Blood Component Separator for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Apheresis Device for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Pathogen Reduction System for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Consumables and Supplies for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Bag and Accessory Blood Transfusion Devices Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By End-user for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Hospital for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Ambulatory Surgical Centres for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Blood Bank for the Period 2021 - 2031
- Historical Data and Forecast of Czech Republic Blood Transfusion Devices Market Revenues & Volume By Other for the Period 2021 - 2031
- Czech Republic Blood Transfusion Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By End-user
- Czech Republic Blood Transfusion Devices Top Companies Market Share
- Czech Republic Blood Transfusion Devices Competitive Benchmarking By Technical and Operational Parameters
- Czech Republic Blood Transfusion Devices Company Profiles
- Czech Republic Blood Transfusion Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Czech Republic Blood Transfusion Devices Market Overview |
3.1 Czech Republic Country Macro Economic Indicators |
3.2 Czech Republic Blood Transfusion Devices Market Revenues & Volume, 2024 & 2031F |
3.3 Czech Republic Blood Transfusion Devices Market - Industry Life Cycle |
3.4 Czech Republic Blood Transfusion Devices Market - Porter's Five Forces |
3.5 Czech Republic Blood Transfusion Devices Market Revenues & Volume Share, By Product, 2024 & 2031F |
3.6 Czech Republic Blood Transfusion Devices Market Revenues & Volume Share, By End-user, 2024 & 2031F |
4 Czech Republic Blood Transfusion Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing demand for blood transfusion services in Czech Republic |
4.2.2 Technological advancements in blood transfusion devices |
4.2.3 Rising prevalence of chronic diseases requiring blood transfusions |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for blood transfusion devices |
4.3.2 High cost associated with blood transfusion devices |
4.3.3 Limited availability of skilled healthcare professionals in the field of blood transfusion |
5 Czech Republic Blood Transfusion Devices Market Trends |
6 Czech Republic Blood Transfusion Devices Market, By Types |
6.1 Czech Republic Blood Transfusion Devices Market, By Product |
6.1.1 Overview and Analysis |
6.1.2 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Product, 2016 - 2031F |
6.1.3 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Blood Bag and Accessory, 2016 - 2031F |
6.1.4 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Blood Mixer, 2016 - 2031F |
6.1.5 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Filter, 2016 - 2031F |
6.1.6 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Blood Component Separator, 2016 - 2031F |
6.1.7 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Apheresis Device, 2016 - 2031F |
6.1.8 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Pathogen Reduction System, 2016 - 2031F |
6.1.9 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Others, 2016 - 2031F |
6.1.10 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Others, 2016 - 2031F |
6.2 Czech Republic Blood Transfusion Devices Market, By End-user |
6.2.1 Overview and Analysis |
6.2.2 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Hospital, 2016 - 2031F |
6.2.3 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Ambulatory Surgical Centres, 2016 - 2031F |
6.2.4 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Blood Bank, 2016 - 2031F |
6.2.5 Czech Republic Blood Transfusion Devices Market Revenues & Volume, By Other, 2016 - 2031F |
7 Czech Republic Blood Transfusion Devices Market Import-Export Trade Statistics |
7.1 Czech Republic Blood Transfusion Devices Market Export to Major Countries |
7.2 Czech Republic Blood Transfusion Devices Market Imports from Major Countries |
8 Czech Republic Blood Transfusion Devices Market Key Performance Indicators |
8.1 Rate of adoption of new blood transfusion devices in Czech Republic |
8.2 Number of blood transfusion procedures performed annually |
8.3 Rate of complications or adverse events related to blood transfusion devices |
9 Czech Republic Blood Transfusion Devices Market - Opportunity Assessment |
9.1 Czech Republic Blood Transfusion Devices Market Opportunity Assessment, By Product, 2024 & 2031F |
9.2 Czech Republic Blood Transfusion Devices Market Opportunity Assessment, By End-user, 2024 & 2031F |
10 Czech Republic Blood Transfusion Devices Market - Competitive Landscape |
10.1 Czech Republic Blood Transfusion Devices Market Revenue Share, By Companies, 2024 |
10.2 Czech Republic Blood Transfusion Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















