France Hydrocortisone Market (2025-2031) Outlook | Value, Revenue, Size, Growth, Companies, Industry, Trends, Share, Analysis & Forecast
| Product Code: ETC328630 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
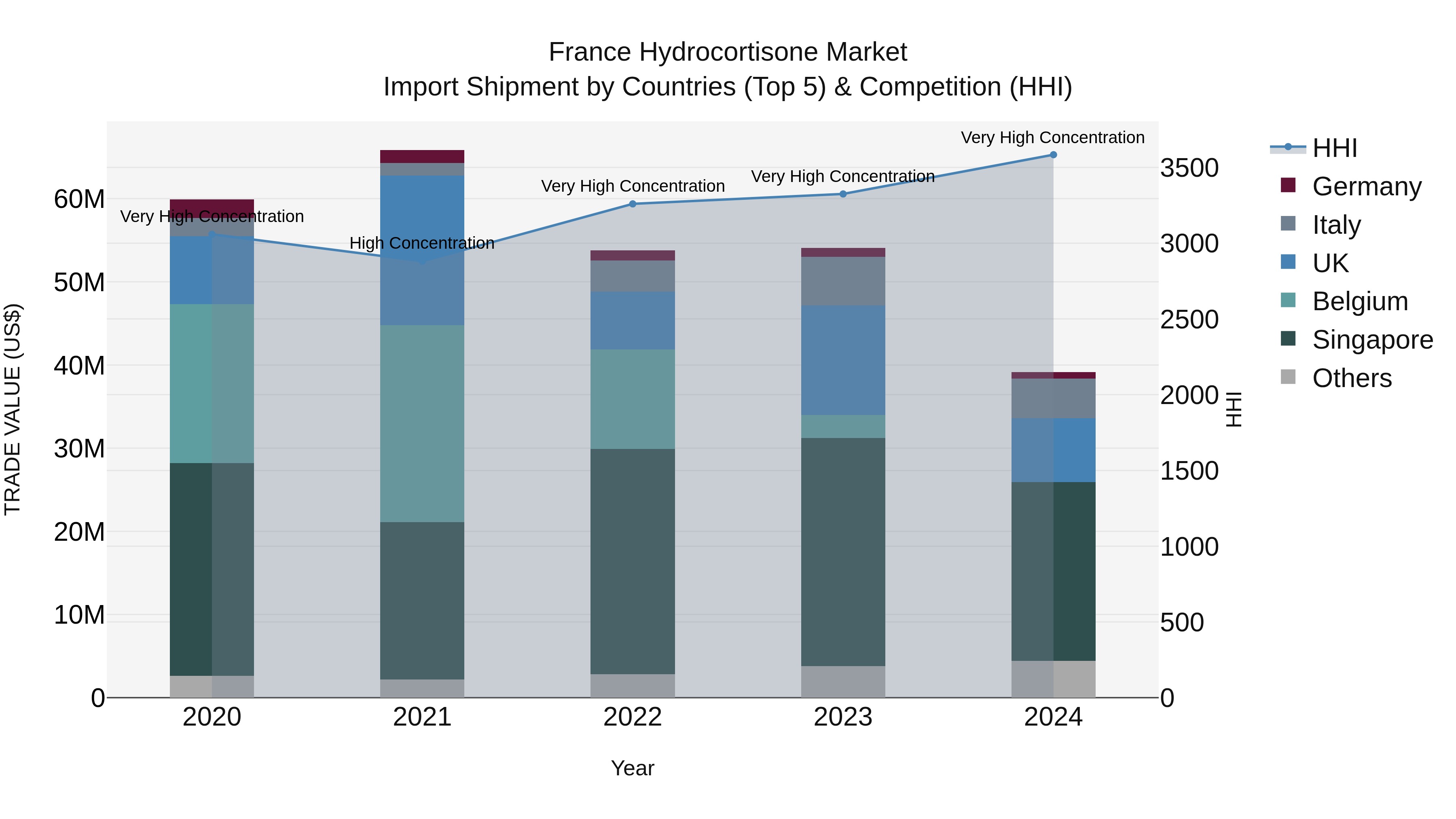
France Hydrocortisone Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s hydrocortisone import market in 2024 continued to show high concentration, with top exporting countries being Singapore, UK, Italy, India, and Germany. However, there was a significant decline in the market size, with a negative compound annual growth rate (CAGR) of -10.09% from 2020 to 2024. The growth rate in 2024 alone plummeted by -27.61%, indicating a challenging year for hydrocortisone imports in France. This data suggests a shifting landscape in the hydrocortisone market that importers and stakeholders should monitor closely for potential opportunities and risks.
France Hydrocortisone Market Synopsis
The France Hydrocortisone Market is a growing sector within the pharmaceutical industry, driven by the increasing prevalence of skin conditions, allergies, and autoimmune diseases among the population. Hydrocortisone, a corticosteroid medication with anti-inflammatory properties, is widely used in the treatment of various skin disorders such as eczema, dermatitis, and psoriasis. The market is characterized by the presence of both generic and branded products, with competition among key players leading to product innovation and competitive pricing strategies. Additionally, the rising awareness about the effectiveness of hydrocortisone in managing skin ailments, coupled with advancements in drug delivery technologies, is expected to further boost market growth in the coming years. Regulatory initiatives aimed at ensuring the quality and safety of hydrocortisone products are also influencing market dynamics in France.
France Hydrocortisone Market Trends
The France Hydrocortisone Market is witnessing a growing demand for hydrocortisone products due to the increasing prevalence of skin conditions such as eczema, psoriasis, and dermatitis. Consumers are increasingly seeking over-the-counter hydrocortisone creams and ointments for managing skin inflammation and irritation. Additionally, the market is experiencing a shift towards natural and organic hydrocortisone alternatives as consumers become more conscious of the ingredients used in skincare products. There is also a rising interest in combination products that offer additional benefits such as moisturizing properties or UV protection. Manufacturers are focusing on product innovation and marketing strategies to differentiate themselves in the competitive market landscape. Overall, the France Hydrocortisone Market is expected to continue growing as consumers prioritize skincare and dermatological health.
France Hydrocortisone Market Challenges
In the France Hydrocortisone market, one of the key challenges faced is increasing competition from generic manufacturers, leading to pricing pressures on branded products. This intensifying competition has resulted in lower profit margins for companies operating in the market. Additionally, regulatory hurdles and stringent approval processes for new hydrocortisone products pose challenges for market entry and product innovation. Moreover, the growing trend of consumer preference towards natural or alternative remedies for skin conditions also presents a hurdle for hydrocortisone products. To succeed in this challenging market landscape, companies need to focus on differentiation strategies, innovation in product offerings, and effective marketing to maintain their market share and profitability.
France Hydrocortisone Market Investment Opportunities
The France Hydrocortisone Market presents several investment opportunities for potential investors. With the increasing prevalence of skin disorders, inflammatory conditions, and autoimmune diseases in the country, the demand for hydrocortisone-based products is expected to rise. Investing in pharmaceutical companies that manufacture and distribute hydrocortisone creams, ointments, and other formulations could be lucrative. Additionally, there is a growing trend towards natural and organic skincare products, creating opportunities for companies developing hydrocortisone alternatives derived from plant-based ingredients. Furthermore, advancements in drug delivery technologies and formulations can also provide avenues for investment in innovative hydrocortisone products with improved efficacy and safety profiles. Overall, the France Hydrocortisone Market offers a diverse range of investment possibilities for those looking to capitalize on the expanding demand for dermatological treatments.
Jordan Agar Market Government Policies
In France, the Hydrocortisone market is regulated by government policies to ensure the safety and efficacy of these medications. The French regulatory agency, Agence Nationale de Sécurité du Médicament (ANSM), oversees the approval, manufacturing, and distribution of Hydrocortisone products. These regulations include requirements for clinical trials, labeling, advertising restrictions, and pharmacovigilance measures to monitor and report any adverse effects. Additionally, pricing and reimbursement policies set by the French government impact the accessibility and affordability of Hydrocortisone drugs for patients. Overall, the government policies in France aim to uphold high standards of quality, safety, and accessibility in the Hydrocortisone market to protect public health.
France Hydrocortisone Market Future Outlook
The France Hydrocortisone market is expected to witness steady growth in the coming years due to increasing awareness and prevalence of skin conditions, allergies, and autoimmune diseases. The rising geriatric population, along with the growing demand for topical corticosteroids for various dermatological conditions, will drive market growth. Additionally, advancements in drug delivery technologies and formulations are likely to enhance the efficacy and safety profile of hydrocortisone products, further fueling market expansion. However, stringent regulations on the use of corticosteroids and the emergence of alternative treatment options may pose challenges to market growth. Overall, the France Hydrocortisone market is poised for moderate growth with opportunities for innovation and market penetration in the forecast period.
Key Highlights of the Report:
- France Hydrocortisone Market Outlook
- Market Size of France Hydrocortisone Market, 2024
- Forecast of France Hydrocortisone Market, 2031
- Historical Data and Forecast of France Hydrocortisone Revenues & Volume for the Period 2021 - 2031
- France Hydrocortisone Market Trend Evolution
- France Hydrocortisone Market Drivers and Challenges
- France Hydrocortisone Price Trends
- France Hydrocortisone Porter's Five Forces
- France Hydrocortisone Industry Life Cycle
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Indication for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Adrenocortical Insufficiency for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Adrenergic Syndrome for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By High Blood Calcium for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Thyroiditis for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Rheumatoid Arthritis for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Dermatitis for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Asthma and Chronic Obstructive Pulmonary Disease (COPD) for the Period 2021 - 2031
- Historical Data and Forecast of France Adrenocortical Insufficiency Hydrocortisone Market Revenues & Volume By Others for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Route of Administration for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Parenteral for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Oral for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Topical for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Distribution Channel for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Hospital Pharmacies for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Retail Pharmacies for the Period 2021 - 2031
- Historical Data and Forecast of France Hydrocortisone Market Revenues & Volume By Online Pharmacies for the Period 2021 - 2031
- France Hydrocortisone Import Export Trade Statistics
- Market Opportunity Assessment By Indication
- Market Opportunity Assessment By Route of Administration
- Market Opportunity Assessment By Distribution Channel
- France Hydrocortisone Top Companies Market Share
- France Hydrocortisone Competitive Benchmarking By Technical and Operational Parameters
- France Hydrocortisone Company Profiles
- France Hydrocortisone Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Hydrocortisone Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Hydrocortisone Market Revenues & Volume, 2021 & 2031F |
3.3 France Hydrocortisone Market - Industry Life Cycle |
3.4 France Hydrocortisone Market - Porter's Five Forces |
3.5 France Hydrocortisone Market Revenues & Volume Share, By Indication, 2021 & 2031F |
3.6 France Hydrocortisone Market Revenues & Volume Share, By Route of Administration, 2021 & 2031F |
3.7 France Hydrocortisone Market Revenues & Volume Share, By Distribution Channel, 2021 & 2031F |
4 France Hydrocortisone Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of skin disorders and autoimmune diseases that require hydrocortisone treatment |
4.2.2 Growing awareness and acceptance of hydrocortisone as an effective treatment option |
4.2.3 Advancements in drug delivery technologies and formulations making hydrocortisone more effective and convenient to use |
4.3 Market Restraints |
4.3.1 Stringent regulations and approvals required for hydrocortisone-based products in France |
4.3.2 Presence of alternative treatment options in the market, impacting the market share of hydrocortisone products |
4.3.3 Concerns regarding potential side effects and long-term use of hydrocortisone products |
5 France Hydrocortisone Market Trends |
6 France Hydrocortisone Market, By Types |
6.1 France Hydrocortisone Market, By Indication |
6.1.1 Overview and Analysis |
6.1.2 France Hydrocortisone Market Revenues & Volume, By Indication, 2021 - 2031F |
6.1.3 France Hydrocortisone Market Revenues & Volume, By Adrenocortical Insufficiency, 2021 - 2031F |
6.1.4 France Hydrocortisone Market Revenues & Volume, By Adrenergic Syndrome, 2021 - 2031F |
6.1.5 France Hydrocortisone Market Revenues & Volume, By High Blood Calcium, 2021 - 2031F |
6.1.6 France Hydrocortisone Market Revenues & Volume, By Thyroiditis, 2021 - 2031F |
6.1.7 France Hydrocortisone Market Revenues & Volume, By Rheumatoid Arthritis, 2021 - 2031F |
6.1.8 France Hydrocortisone Market Revenues & Volume, By Dermatitis, 2021 - 2031F |
6.1.9 France Hydrocortisone Market Revenues & Volume, By Others, 2021 - 2031F |
6.1.10 France Hydrocortisone Market Revenues & Volume, By Others, 2021 - 2031F |
6.2 France Hydrocortisone Market, By Route of Administration |
6.2.1 Overview and Analysis |
6.2.2 France Hydrocortisone Market Revenues & Volume, By Parenteral, 2021 - 2031F |
6.2.3 France Hydrocortisone Market Revenues & Volume, By Oral, 2021 - 2031F |
6.2.4 France Hydrocortisone Market Revenues & Volume, By Topical, 2021 - 2031F |
6.3 France Hydrocortisone Market, By Distribution Channel |
6.3.1 Overview and Analysis |
6.3.2 France Hydrocortisone Market Revenues & Volume, By Hospital Pharmacies, 2021 - 2031F |
6.3.3 France Hydrocortisone Market Revenues & Volume, By Retail Pharmacies, 2021 - 2031F |
6.3.4 France Hydrocortisone Market Revenues & Volume, By Online Pharmacies, 2021 - 2031F |
7 France Hydrocortisone Market Import-Export Trade Statistics |
7.1 France Hydrocortisone Market Export to Major Countries |
7.2 France Hydrocortisone Market Imports from Major Countries |
8 France Hydrocortisone Market Key Performance Indicators |
8.1 Patient adherence rate to hydrocortisone treatment |
8.2 Number of new product launches and innovations in hydrocortisone formulations |
8.3 Prescription rates by dermatologists for hydrocortisone products |
9 France Hydrocortisone Market - Opportunity Assessment |
9.1 France Hydrocortisone Market Opportunity Assessment, By Indication, 2021 & 2031F |
9.2 France Hydrocortisone Market Opportunity Assessment, By Route of Administration, 2021 & 2031F |
9.3 France Hydrocortisone Market Opportunity Assessment, By Distribution Channel, 2021 & 2031F |
10 France Hydrocortisone Market - Competitive Landscape |
10.1 France Hydrocortisone Market Revenue Share, By Companies, 2024 |
10.2 France Hydrocortisone Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















