France Osteosynthesis Devices Market (2025-2031) | Segmentation, Trends, Size & Revenue, Value, Analysis, Forecast, Share, Industry, Outlook, Competitive Landscape, Growth, Companies
| Product Code: ETC7225480 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
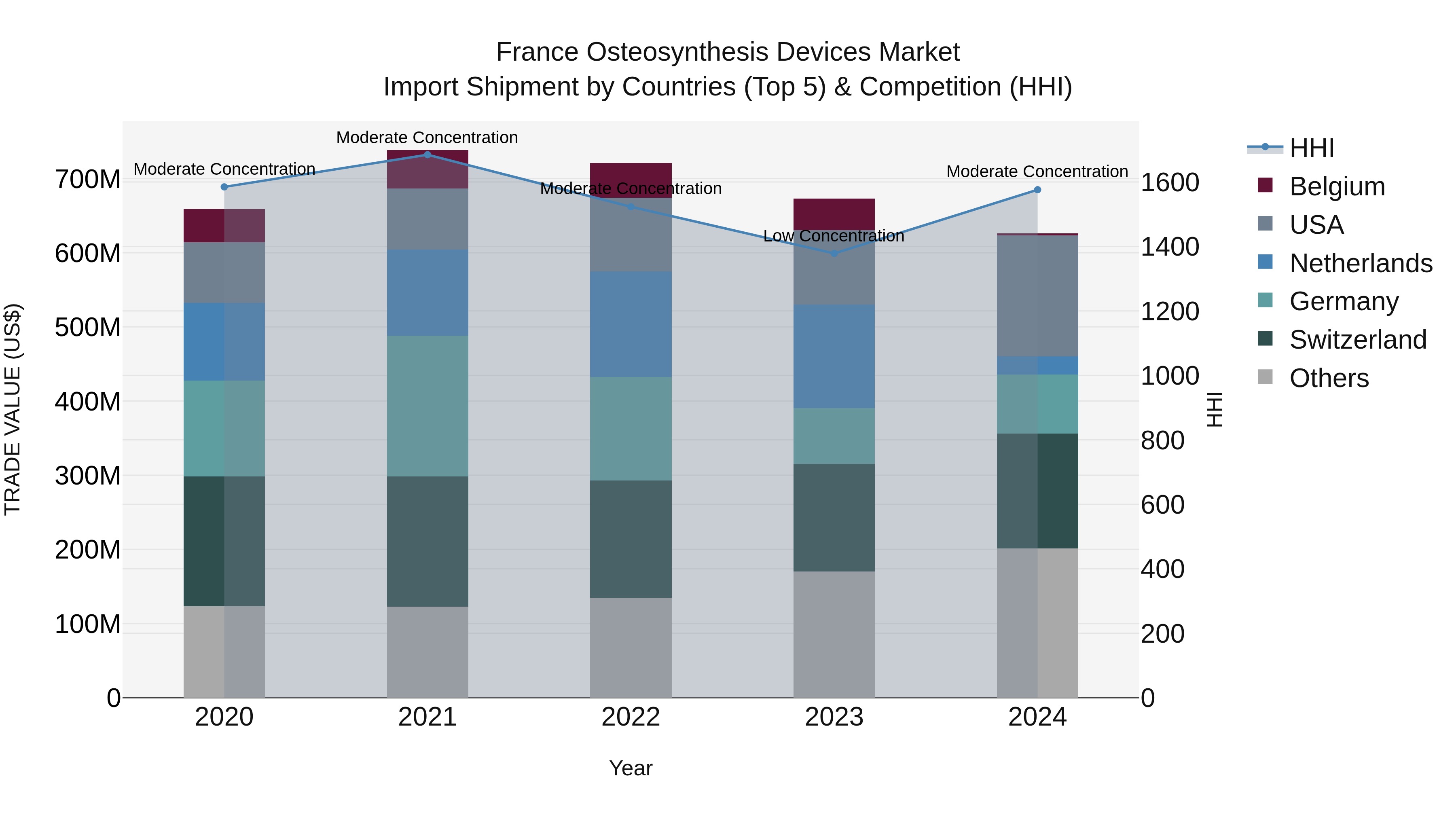
France Osteosynthesis Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
France`s import shipments of osteosynthesis devices in 2024 show a shift in concentration, moving from low to moderate concentration as per the HHI index. The top exporting countries to France include the USA, Switzerland, Germany, Mexico, and China, indicating a diverse source of imports. However, the market experienced a decline with a negative CAGR of -1.28% from 2020-24 and a further decrease in growth rate of -7.02% from 2023-24. This data suggests a challenging market environment for osteosynthesis device imports in France.
France Osteosynthesis Devices Market Overview
The France Osteosynthesis Devices Market is a significant segment within the medical device industry, encompassing a range of implants and instruments used in orthopedic surgeries to stabilize and repair bone fractures and deformities. Key players in the market include major international companies such as DePuy Synthes, Stryker Corporation, and Zimmer Biomet, as well as various local manufacturers. The market is driven by factors such as the increasing prevalence of musculoskeletal disorders, rising geriatric population, and advancements in surgical techniques. France`s strong healthcare infrastructure, skilled orthopedic surgeons, and growing investments in healthcare further contribute to the market`s growth. However, challenges such as stringent regulations, pricing pressures, and competition from alternative treatment options are also present, influencing market dynamics and strategies of industry players.
France Osteosynthesis Devices Market Trends and Opportunities
The France Osteosynthesis Devices Market is witnessing a growing trend towards minimally invasive procedures, driven by advancements in technology and increasing demand for quicker recovery times. Additionally, the market is experiencing a rise in the adoption of biodegradable implants, as they offer the advantage of reducing the risk of infections and eliminating the need for a second surgery for implant removal. Opportunities in the market include the development of patient-specific implants through 3D printing technology, which can improve surgical outcomes and reduce complications. Furthermore, the increasing prevalence of orthopedic injuries and fractures among the aging population in France presents a significant opportunity for market growth, as the demand for osteosynthesis devices is expected to increase in the coming years.
France Osteosynthesis Devices Market Challenges
In the France Osteosynthesis Devices Market, key challenges include regulatory hurdles and compliance requirements, intense competition among market players leading to pricing pressures, and the need for continuous innovation to meet evolving patient needs and technological advancements. Additionally, the market faces challenges related to reimbursement policies, healthcare budget constraints, and the impact of economic fluctuations on healthcare spending. Maintaining high-quality standards while keeping costs under control is a constant challenge for companies operating in this market. Furthermore, the market dynamics are influenced by factors such as changing demographics, increasing prevalence of orthopedic conditions, and the shift towards minimally invasive procedures, requiring companies to adapt quickly to stay competitive in the evolving landscape.
France Osteosynthesis Devices Market Drivers
The France Osteosynthesis Devices Market is primarily driven by factors such as the increasing prevalence of orthopedic disorders and injuries, rising geriatric population, and advancements in surgical techniques and technologies. The growing number of road accidents and sports-related injuries also contribute to the demand for osteosynthesis devices in France. Additionally, the expanding healthcare infrastructure, rising healthcare expenditure, and a growing focus on minimally invasive procedures further propel market growth. The availability of innovative products, such as bioresorbable implants and customized implants, also play a significant role in driving the market forward. Overall, the increasing awareness about the benefits of early intervention and improved patient outcomes are key factors stimulating the growth of the France Osteosynthesis Devices Market.
France Osteosynthesis Devices Market Government Policies
The France Osteosynthesis Devices Market is subject to various government policies aimed at regulating the medical device industry. These policies include the European Medical Devices Regulation (MDR) which sets out requirements for the safety and performance of medical devices, including osteosynthesis devices. Additionally, the French National Agency for the Safety of Medicines and Health Products (ANSM) oversees the market authorization and surveillance of medical devices in France, ensuring compliance with regulatory standards. The government also plays a role in determining reimbursement policies for osteosynthesis devices through the national healthcare system, which can impact market access and pricing strategies for manufacturers operating in the French market. Overall, these government policies aim to ensure the safety, efficacy, and quality of osteosynthesis devices available in France while maintaining transparency and regulatory compliance within the market.
France Osteosynthesis Devices Market Future Outlook
The France Osteosynthesis Devices Market is expected to witness steady growth in the coming years due to factors such as the rising incidence of orthopedic disorders, increasing geriatric population, and advancements in medical technology. The market is likely to be driven by the demand for minimally invasive procedures, innovative implant materials, and personalized treatment options. Additionally, the growing emphasis on enhancing surgical outcomes and reducing hospital stays is projected to fuel the adoption of osteosynthesis devices in France. With ongoing research and development activities focusing on improving the efficacy and safety of these devices, the market is poised for expansion. However, pricing pressures, stringent regulatory requirements, and competition among key players may pose challenges for market growth in the future.
Key Highlights of the Report:
- France Osteosynthesis Devices Market Outlook
- Market Size of France Osteosynthesis Devices Market, 2024
- Forecast of France Osteosynthesis Devices Market, 2031
- Historical Data and Forecast of France Osteosynthesis Devices Revenues & Volume for the Period 2021- 2031
- France Osteosynthesis Devices Market Trend Evolution
- France Osteosynthesis Devices Market Drivers and Challenges
- France Osteosynthesis Devices Price Trends
- France Osteosynthesis Devices Porter's Five Forces
- France Osteosynthesis Devices Industry Life Cycle
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Fracture Type for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Hip Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Knee Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Spine Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Shoulder Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Foot and Ankle Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Facial bones Fracture for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By End User for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Orthopaedic Clinics for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Ambulatory surgical Centers for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Device Type for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By External Fixation Devices for the Period 2021- 2031
- Historical Data and Forecast of France Osteosynthesis Devices Market Revenues & Volume By Internal Fixation Devices for the Period 2021- 2031
- France Osteosynthesis Devices Import Export Trade Statistics
- Market Opportunity Assessment By Fracture Type
- Market Opportunity Assessment By End User
- Market Opportunity Assessment By Device Type
- France Osteosynthesis Devices Top Companies Market Share
- France Osteosynthesis Devices Competitive Benchmarking By Technical and Operational Parameters
- France Osteosynthesis Devices Company Profiles
- France Osteosynthesis Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 France Osteosynthesis Devices Market Overview |
3.1 France Country Macro Economic Indicators |
3.2 France Osteosynthesis Devices Market Revenues & Volume, 2021 & 2031F |
3.3 France Osteosynthesis Devices Market - Industry Life Cycle |
3.4 France Osteosynthesis Devices Market - Porter's Five Forces |
3.5 France Osteosynthesis Devices Market Revenues & Volume Share, By Fracture Type, 2021 & 2031F |
3.6 France Osteosynthesis Devices Market Revenues & Volume Share, By End User, 2021 & 2031F |
3.7 France Osteosynthesis Devices Market Revenues & Volume Share, By Device Type, 2021 & 2031F |
4 France Osteosynthesis Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing incidence of orthopedic conditions and fractures in France |
4.2.2 Technological advancements leading to the development of innovative osteosynthesis devices |
4.2.3 Growing demand for minimally invasive surgeries in the country |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for approval of osteosynthesis devices in France |
4.3.2 High cost associated with osteosynthesis procedures and devices |
4.3.3 Limited reimbursement policies for osteosynthesis surgeries in the market |
5 France Osteosynthesis Devices Market Trends |
6 France Osteosynthesis Devices Market, By Types |
6.1 France Osteosynthesis Devices Market, By Fracture Type |
6.1.1 Overview and Analysis |
6.1.2 France Osteosynthesis Devices Market Revenues & Volume, By Fracture Type, 2021- 2031F |
6.1.3 France Osteosynthesis Devices Market Revenues & Volume, By Hip Fracture, 2021- 2031F |
6.1.4 France Osteosynthesis Devices Market Revenues & Volume, By Knee Fracture, 2021- 2031F |
6.1.5 France Osteosynthesis Devices Market Revenues & Volume, By Spine Fracture, 2021- 2031F |
6.1.6 France Osteosynthesis Devices Market Revenues & Volume, By Shoulder Fracture, 2021- 2031F |
6.1.7 France Osteosynthesis Devices Market Revenues & Volume, By Foot and Ankle Fracture, 2021- 2031F |
6.1.8 France Osteosynthesis Devices Market Revenues & Volume, By Facial bones Fracture, 2021- 2031F |
6.2 France Osteosynthesis Devices Market, By End User |
6.2.1 Overview and Analysis |
6.2.2 France Osteosynthesis Devices Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.2.3 France Osteosynthesis Devices Market Revenues & Volume, By Orthopaedic Clinics, 2021- 2031F |
6.2.4 France Osteosynthesis Devices Market Revenues & Volume, By Ambulatory surgical Centers, 2021- 2031F |
6.3 France Osteosynthesis Devices Market, By Device Type |
6.3.1 Overview and Analysis |
6.3.2 France Osteosynthesis Devices Market Revenues & Volume, By External Fixation Devices, 2021- 2031F |
6.3.3 France Osteosynthesis Devices Market Revenues & Volume, By Internal Fixation Devices, 2021- 2031F |
7 France Osteosynthesis Devices Market Import-Export Trade Statistics |
7.1 France Osteosynthesis Devices Market Export to Major Countries |
7.2 France Osteosynthesis Devices Market Imports from Major Countries |
8 France Osteosynthesis Devices Market Key Performance Indicators |
8.1 Average length of hospital stay for patients undergoing osteosynthesis procedures |
8.2 Adoption rate of minimally invasive osteosynthesis techniques |
8.3 Number of research and development collaborations in the osteosynthesis devices market in France |
9 France Osteosynthesis Devices Market - Opportunity Assessment |
9.1 France Osteosynthesis Devices Market Opportunity Assessment, By Fracture Type, 2021 & 2031F |
9.2 France Osteosynthesis Devices Market Opportunity Assessment, By End User, 2021 & 2031F |
9.3 France Osteosynthesis Devices Market Opportunity Assessment, By Device Type, 2021 & 2031F |
10 France Osteosynthesis Devices Market - Competitive Landscape |
10.1 France Osteosynthesis Devices Market Revenue Share, By Companies, 2024 |
10.2 France Osteosynthesis Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
Export potential assessment - trade Analytics for 2030
Export potential enables firms to identify high-growth global markets with greater confidence by combining advanced trade intelligence with a structured quantitative methodology. The framework analyzes emerging demand trends and country-level import patterns while integrating macroeconomic and trade datasets such as GDP and population forecasts, bilateral import–export flows, tariff structures, elasticity differentials between developed and developing economies, geographic distance, and import demand projections. Using weighted trade values from 2020–2024 as the base period to project country-to-country export potential for 2030, these inputs are operationalized through calculated drivers such as gravity model parameters, tariff impact factors, and projected GDP per-capita growth. Through an analysis of hidden potentials, demand hotspots, and market conditions that are most favorable to success, this method enables firms to focus on target countries, maximize returns, and global expansion with data, backed by accuracy.
By factoring in the projected importer demand gap that is currently unmet and could be potential opportunity, it identifies the potential for the Exporter (Country) among 190 countries, against the general trade analysis, which identifies the biggest importer or exporter.
To discover high-growth global markets and optimize your business strategy:
Click Here- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- India Switchgear Market Outlook (2026 - 2032) | Size, Share, Trends, Growth, Revenue, Forecast, Analysis, Value, Outlook
- Pakistan Contraceptive Implants Market (2025-2031) | Demand, Growth, Size, Share, Industry, Pricing Analysis, Competitive, Strategic Insights, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Companies, Challenges
- Sri Lanka Packaging Market (2026-2032) | Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints
- India Kids Watches Market (2026-2032) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Saudi Arabia Core Assurance Service Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Romania Uninterruptible Power Supply (UPS) Market (2026-2032) | Industry, Analysis, Revenue, Size, Forecast, Outlook, Value, Trends, Share, Growth & Companies
- Saudi Arabia Car Window Tinting Film, Paint Protection Film (PPF), and Ceramic Coating Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















