Latvia Endoscopy Operative Devices Market (2025-2031) | Competitive Landscape, Industry, Analysis, Value, Trends, Forecast, Outlook, Segmentation, Share, Companies, Growth, Size & Revenue
| Product Code: ETC7910397 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Vasudha | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Latvia Endoscopy Operative Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, Latvia continued to rely heavily on imports of endoscopy operative devices, with top exporters being Germany, Netherlands, Lithuania, China, and Ireland. The market showed high concentration levels, indicating limited supplier diversity. Despite a moderate compound annual growth rate (CAGR) of 2.97% from 2020 to 2024, there was a significant decline in growth rate from 2023 to 2024 at -17.96%. This fluctuation suggests a need for market players to adapt to changing dynamics and potentially explore new strategies to stimulate demand and drive growth in the Latvian endoscopy device market.
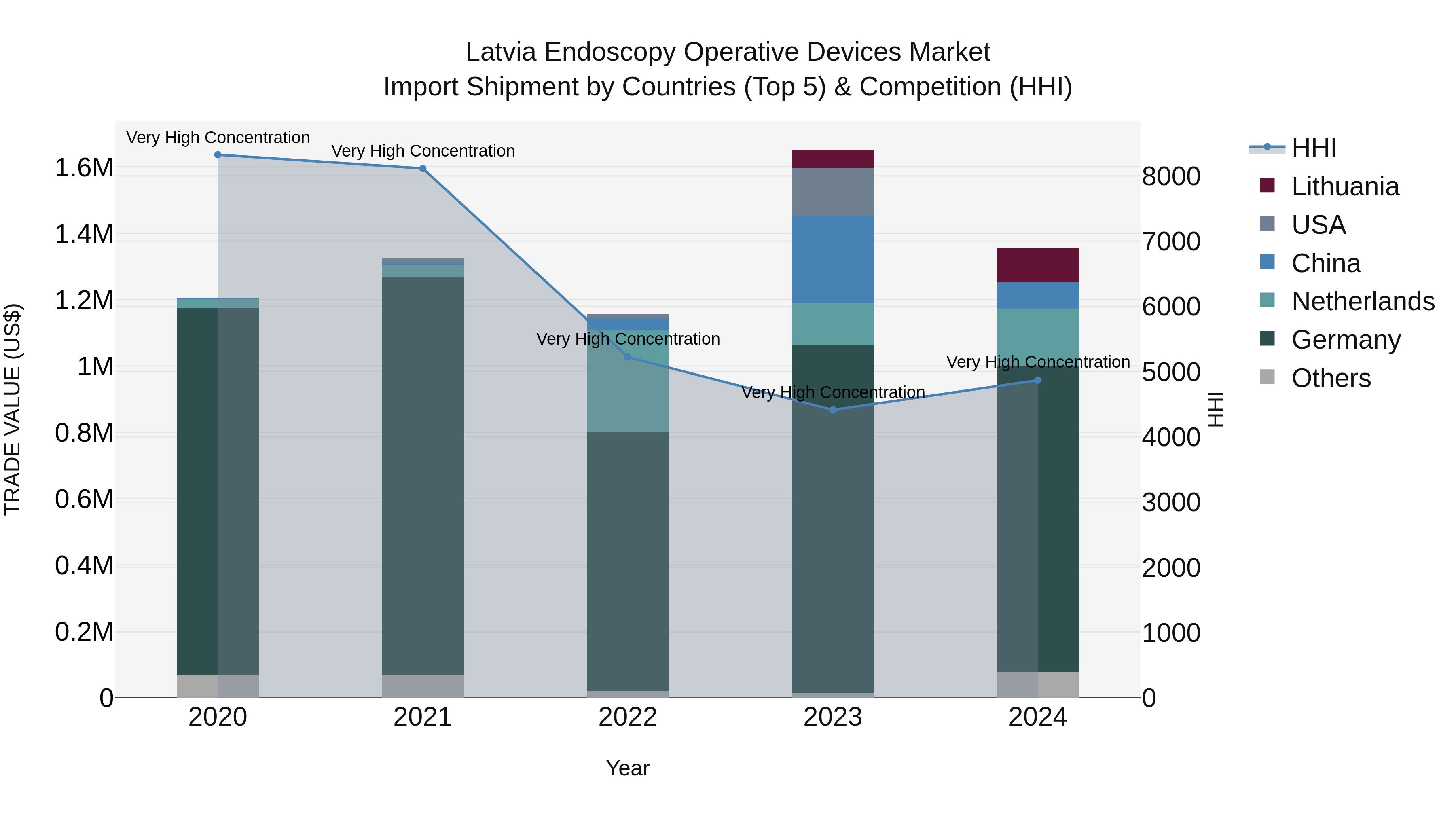
Latvia Endoscopy Operative Devices Market Overview
The Latvia Endoscopy Operative Devices Market is experiencing steady growth driven by advancements in medical technology and increasing demand for minimally invasive procedures. Key players in the market include companies like Olympus Corporation, Karl Storz GmbH & Co. KG, and Medtronic plc. The market offers a wide range of endoscopy operative devices such as endoscopes, trocars, graspers, and energy devices used in various surgical procedures. Rising healthcare expenditure, growing awareness about the benefits of endoscopic surgeries, and the presence of well-established healthcare infrastructure are contributing to the market`s expansion. Additionally, favorable reimbursement policies and a rising prevalence of gastrointestinal diseases are further propelling the demand for endoscopy operative devices in Latvia. The market is poised for continued growth as healthcare facilities continue to adopt advanced endoscopic technologies for better patient outcomes.
Latvia Endoscopy Operative Devices Market Trends and Opportunities
The Latvia Endoscopy Operative Devices Market is experiencing significant growth due to the increasing prevalence of gastrointestinal disorders and the rising adoption of minimally invasive surgical procedures. Key trends in the market include the development of advanced endoscopic technologies such as high-definition imaging systems, flexible endoscopes, and robotic-assisted surgical devices. There is a growing demand for disposable and single-use endoscopic instruments to prevent cross-contamination and reduce healthcare-associated infections. Opportunities in the market lie in expanding partnerships between medical device manufacturers and healthcare providers to improve access to endoscopy services in remote areas, as well as the introduction of innovative products tailored to specific clinical needs. Overall, the market is poised for continued expansion driven by advancements in technology and the growing preference for minimally invasive procedures.
Latvia Endoscopy Operative Devices Market Challenges
In the Latvia Endoscopy Operative Devices Market, some key challenges include limited access to advanced technology and equipment, high costs associated with purchasing and maintaining endoscopy devices, and a shortage of skilled healthcare professionals trained in using these complex devices. Additionally, regulatory hurdles and reimbursement issues may also hinder market growth. The relatively small market size and competition from established global players further pose challenges for local companies trying to penetrate the market. Overall, overcoming these obstacles requires strategic partnerships, investments in training programs, and efforts to streamline regulatory processes to foster innovation and growth in the Latvia Endoscopy Operative Devices Market.
Latvia Endoscopy Operative Devices Market Drivers
The Latvia Endoscopy Operative Devices Market is primarily driven by the increasing prevalence of gastrointestinal disorders and the growing demand for minimally invasive procedures. The rising adoption of advanced endoscopic technologies, such as HD imaging and robotic-assisted systems, is also fueling market growth. Additionally, favorable reimbursement policies, ongoing technological advancements in endoscopy devices, and a surge in healthcare infrastructure development are contributing to the expansion of the market. Furthermore, the growing awareness among both healthcare professionals and patients about the benefits of endoscopic procedures in terms of reduced recovery time, minimal scarring, and lower risk of complications is driving the demand for endoscopy operative devices in Latvia.
Latvia Endoscopy Operative Devices Market Government Policy
In Latvia, the regulation of endoscopy operative devices falls under the supervision of the State Agency of Medicines (ZVA). The agency is responsible for ensuring that these medical devices meet the necessary safety and quality standards before they can be sold or used in the country. Endoscopy devices are classified as medical devices, and their registration, marketing, and post-market surveillance are governed by the Medical Devices Law. Manufacturers and distributors of these devices must comply with the requirements set forth by the European Union Medical Devices Regulation (MDR) to ensure patient safety and device effectiveness. Additionally, the Latvian government has implemented measures to promote the use of advanced endoscopy technologies in healthcare facilities to improve patient outcomes and enhance the quality of medical services in the country.
Latvia Endoscopy Operative Devices Market Future Outlook
The future outlook for the Latvia Endoscopy Operative Devices Market appears promising, driven by factors such as the increasing prevalence of gastrointestinal disorders, technological advancements in endoscopic procedures, and a growing emphasis on minimally invasive surgeries. The market is expected to witness steady growth as healthcare facilities invest in advanced endoscopy equipment to improve diagnostic and therapeutic capabilities. Additionally, rising awareness among both patients and healthcare professionals about the benefits of endoscopy procedures is likely to contribute to market expansion. However, challenges such as high equipment costs and limited reimbursement policies may hinder the market growth to some extent. Overall, with the continual innovation in endoscopic technologies and increasing demand for minimally invasive procedures, the Latvia Endoscopy Operative Devices Market is projected to experience positive growth in the coming years.
Key Highlights of the Report:
- Latvia Endoscopy Operative Devices Market Outlook
- Market Size of Latvia Endoscopy Operative Devices Market, 2024
- Forecast of Latvia Endoscopy Operative Devices Market, 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Revenues & Volume for the Period 2021- 2031
- Latvia Endoscopy Operative Devices Market Trend Evolution
- Latvia Endoscopy Operative Devices Market Drivers and Challenges
- Latvia Endoscopy Operative Devices Price Trends
- Latvia Endoscopy Operative Devices Porter's Five Forces
- Latvia Endoscopy Operative Devices Industry Life Cycle
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Energy Systems for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Access Devices for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Suction & Irrigation Systems for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Hand Instruments for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Wound Retractors for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Snares for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Gastrointestinal (GI) Endoscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Bronchoscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Obstetrics/Gynecology Endoscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Arthroscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Laparoscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Urology Endoscopy (Cystoscopy) for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Mediastinoscopy for the Period 2021- 2031
- Historical Data and Forecast of Latvia Endoscopy Operative Devices Market Revenues & Volume By Others for the Period 2021- 2031
- Latvia Endoscopy Operative Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Application
- Latvia Endoscopy Operative Devices Top Companies Market Share
- Latvia Endoscopy Operative Devices Competitive Benchmarking By Technical and Operational Parameters
- Latvia Endoscopy Operative Devices Company Profiles
- Latvia Endoscopy Operative Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















