Netherlands Orthopedic Biomaterials Market (2025-2031) | Regulations, Market Penetration, Consumer Insights, Opportunities, Value, Companies, Landscape, Technological Advancements, Analysis, Segments, Segmentation, Future Prospects, Strategic Insights, Industry, Competitive Landscape, Investment Trends, Challenges, Share, Competition, Growth, Pricing Analysis, Trends, Drivers, Size, Innovation, Strategy, Revenue, Restraints, Outlook, Demand, Supply, Forecast
| Product Code: ETC10738764 | Publication Date: Apr 2025 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 65 | No. of Figures: 34 | No. of Tables: 19 |
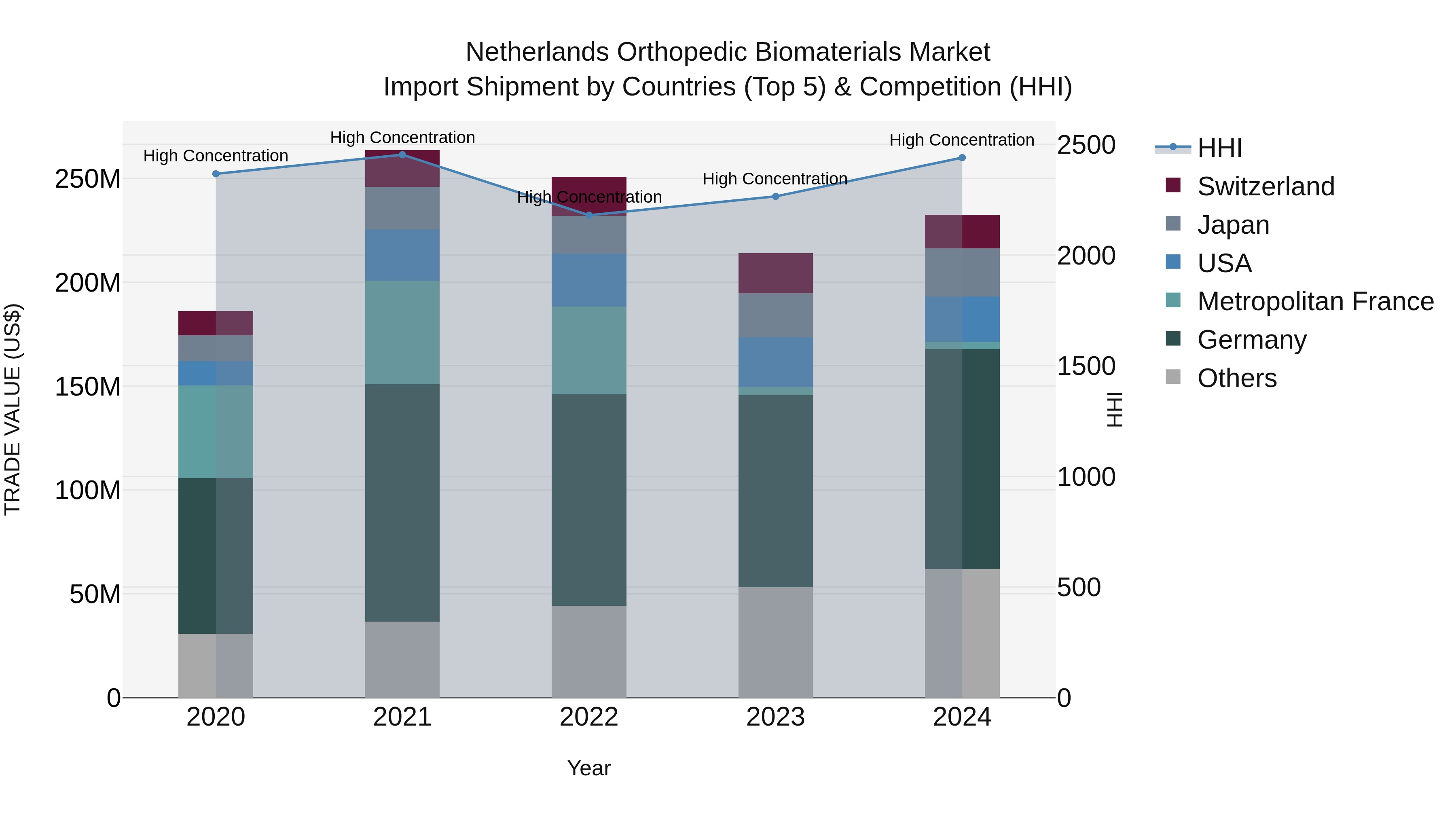
Netherlands Orthopedic Biomaterials Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The Netherlands continues to be a key destination for orthopedic biomaterial imports, with top exporters like Germany, Japan, and the USA dominating the market in 2024. The high Herfindahl-Hirschman Index (HHI) indicates a concentrated market, while the impressive Compound Annual Growth Rate (CAGR) of 5.72% from 2020-2024 highlights sustained growth. The notable growth rate of 8.68% from 2023-2024 signals a rapidly expanding market, making the Netherlands a lucrative hub for orthopedic biomaterial imports.
Netherlands Orthopedic Biomaterials Market Overview
The Netherlands orthopedic biomaterials market is experiencing steady growth, driven by an aging population, rising incidence of musculoskeletal disorders, and advancements in biomaterial technologies. The market encompasses products such as bone grafts, bone substitutes, polymers, ceramics, and metallic biomaterials, primarily used in joint reconstruction, fracture fixation, and spinal surgeries. Leading global and regional players, including Stryker, Zimmer Biomet, and local innovators, are active in the market, fostering competition and innovation. The countryâs well-developed healthcare infrastructure, high adoption of advanced orthopedic procedures, and supportive reimbursement policies further support market expansion. However, challenges such as stringent regulatory requirements and cost pressures persist. Growing investments in R&D, a focus on minimally invasive surgeries, and increasing collaborations between research institutions and manufacturers are expected to drive future growth, positioning the Netherlands as a key hub for orthopedic biomaterials in Europe.
Trends of the Market
The Netherlands orthopedic biomaterials market is experiencing steady growth, driven by an aging population, rising incidences of musculoskeletal disorders, and increasing demand for minimally invasive surgical procedures. Recent trends include a shift towards bioresorbable materials and advanced composites that promote faster healing and reduce the risk of post-surgical complications. There is a notable emphasis on personalized and 3D-printed implants tailored to individual patient needs. Additionally, biocompatible materials that enhance osseointegration are gaining traction, supported by ongoing research collaborations between leading universities and medical device companies. Regulatory focus on safety and sustainable manufacturing is encouraging innovation in eco-friendly biomaterials. Overall, the market is characterized by technological advancements and a strong commitment to improving patient outcomes.
Challenges of the Market
The Netherlands orthopedic biomaterials market faces several challenges, including stringent regulatory requirements that prolong product approval timelines and increase costs for manufacturers. Intense competition from both international and local players puts pressure on pricing and margins, while the high cost of advanced biomaterial implants limits accessibility and reimbursement from healthcare providers. Additionally, there is a slow adoption rate of novel biomaterials among clinicians due to concerns about long-term safety and efficacy, compounded by limited clinical data. The aging population, though increasing demand, also raises the prevalence of comorbidities that complicate implant procedures and outcomes. Furthermore, supply chain disruptions and shortages of raw materials, exacerbated by global events, hinder consistent market growth.
Investment Opportunities of the market
The Netherlands orthopedic biomaterials market offers promising investment opportunities driven by an aging population, rising incidence of musculoskeletal disorders, and an advanced healthcare infrastructure. Demand is increasing for innovative biomaterials such as bioresorbable polymers, bone graft substitutes, and 3D-printed implants, spurred by a focus on minimally invasive surgeries and faster patient recovery. The countryâs strong R&D ecosystem, supported by prominent universities and medical technology clusters, encourages partnerships and the development of next-generation products. Regulatory alignment with the EU and a high adoption rate of novel medical technologies further accelerate market entry. Companies investing in local manufacturing, sustainable biomaterials, and digital health integrationâsuch as smart implants and remote monitoringâcan capitalize on the evolving needs of hospitals and orthopedic clinics, making the Netherlands a strategic hub for expansion in the orthopedic biomaterials sector.
Government Policy of the market
The Netherlands` government supports the orthopedic biomaterials market through stringent regulatory frameworks and innovation-driven policies. The Dutch Medicines Evaluation Board (CBG) and the Health and Youth Care Inspectorate (IGJ) oversee the safety and quality of medical devices, including orthopedic biomaterials, in alignment with the European Union Medical Device Regulation (EU MDR). These regulations require rigorous clinical evaluations, post-market surveillance, and compliance with safety standards. Additionally, the government promotes public-private partnerships and research funding through organizations like the Netherlands Organisation for Health Research and Development (ZonMw), encouraging innovation and adoption of advanced biomaterials. Reimbursement policies are managed by the Dutch Healthcare Authority (NZa), ensuring that cost-effective, evidence-based biomaterial solutions are accessible. Collectively, these policies foster a secure, innovative, and well-regulated market environment for orthopedic biomaterials in the Netherlands.
Future Outlook of the market
The future outlook for the Netherlands Orthopedic Biomaterials Market is promising, driven by the countryâs aging population, increasing prevalence of musculoskeletal disorders, and advancements in biomaterial technologies. Rising demand for minimally invasive surgical procedures and a strong healthcare infrastructure support market growth. Innovative biomaterials, such as bioresorbable polymers and ceramics, are gaining traction due to improved patient outcomes and faster recovery times. Furthermore, supportive government initiatives and investments in research and development are fostering innovation and attracting global players. Despite challenges like regulatory complexities and high costs, the market is expected to expand steadily over the next five years, with orthopedic implants, bone graft substitutes, and regenerative biomaterials being key growth segments.
Key Highlights of the Report:
- Netherlands Orthopedic Biomaterials Market Outlook
- Market Size of Netherlands Orthopedic Biomaterials Market,2024
- Forecast of Netherlands Orthopedic Biomaterials Market, 2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Revenues & Volume for the Period 2022-2031
- Netherlands Orthopedic Biomaterials Market Trend Evolution
- Netherlands Orthopedic Biomaterials Market Drivers and Challenges
- Netherlands Orthopedic Biomaterials Price Trends
- Netherlands Orthopedic Biomaterials Porter's Five Forces
- Netherlands Orthopedic Biomaterials Industry Life Cycle
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Material Type for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Metallic Biomaterials for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Polymer-Based for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Ceramic-Based for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Natural Biomaterials for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Application for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Joint Replacement for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Spine Surgery for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Trauma Fixation for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Sports Medicine for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By End User for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Hospitals for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Clinics for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Ambulatory Centers for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Research Institutes for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Others for the Period 2021 - 2029
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Product Form for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Powder for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Liquid for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Solid for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Gel for the Period 2022-2031
- Historical Data and Forecast of Netherlands Orthopedic Biomaterials Market Revenues & Volume By Others for the Period 2021 - 2029
- Netherlands Orthopedic Biomaterials Import Export Trade Statistics
- Market Opportunity Assessment By Material Type
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End User
- Market Opportunity Assessment By Product Form
- Netherlands Orthopedic Biomaterials Top Companies Market Share
- Netherlands Orthopedic Biomaterials Competitive Benchmarking By Technical and Operational Parameters
- Netherlands Orthopedic Biomaterials Company Profiles
- Netherlands Orthopedic Biomaterials Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Netherlands Orthopedic Biomaterials Market Overview |
3.1 Netherlands Country Macro Economic Indicators |
3.2 Netherlands Orthopedic Biomaterials Market Revenues & Volume, 2024 & 2031F |
3.3 Netherlands Orthopedic Biomaterials Market - Industry Life Cycle |
3.4 Netherlands Orthopedic Biomaterials Market - Porter's Five Forces |
3.5 Netherlands Orthopedic Biomaterials Market Revenues & Volume Share, By Material Type, 2024 & 2031F |
3.6 Netherlands Orthopedic Biomaterials Market Revenues & Volume Share, By Application, 2024 & 2031F |
3.7 Netherlands Orthopedic Biomaterials Market Revenues & Volume Share, By End User, 2024 & 2031F |
3.8 Netherlands Orthopedic Biomaterials Market Revenues & Volume Share, By Product Form, 2024 & 2031F |
4 Netherlands Orthopedic Biomaterials Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.3 Market Restraints |
5 Netherlands Orthopedic Biomaterials Market Trends |
6 Netherlands Orthopedic Biomaterials Market, By Types |
6.1 Netherlands Orthopedic Biomaterials Market, By Material Type |
6.1.1 Overview and Analysis |
6.1.2 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Material Type, 2022 - 2031F |
6.1.3 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Metallic Biomaterials, 2022 - 2031F |
6.1.4 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Polymer-Based, 2022 - 2031F |
6.1.5 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Ceramic-Based, 2022 - 2031F |
6.1.6 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Natural Biomaterials, 2022 - 2031F |
6.1.7 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Others, 2022 - 2031F |
6.2 Netherlands Orthopedic Biomaterials Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Joint Replacement, 2022 - 2031F |
6.2.3 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Spine Surgery, 2022 - 2031F |
6.2.4 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Trauma Fixation, 2022 - 2031F |
6.2.5 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Sports Medicine, 2022 - 2031F |
6.2.6 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Others, 2022 - 2031F |
6.3 Netherlands Orthopedic Biomaterials Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Hospitals, 2022 - 2031F |
6.3.3 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Clinics, 2022 - 2031F |
6.3.4 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Ambulatory Centers, 2022 - 2031F |
6.3.5 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Research Institutes, 2022 - 2031F |
6.3.6 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Others, 2022 - 2031F |
6.4 Netherlands Orthopedic Biomaterials Market, By Product Form |
6.4.1 Overview and Analysis |
6.4.2 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Powder, 2022 - 2031F |
6.4.3 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Liquid, 2022 - 2031F |
6.4.4 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Solid, 2022 - 2031F |
6.4.5 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Gel, 2022 - 2031F |
6.4.6 Netherlands Orthopedic Biomaterials Market Revenues & Volume, By Others, 2022 - 2031F |
7 Netherlands Orthopedic Biomaterials Market Import-Export Trade Statistics |
7.1 Netherlands Orthopedic Biomaterials Market Export to Major Countries |
7.2 Netherlands Orthopedic Biomaterials Market Imports from Major Countries |
8 Netherlands Orthopedic Biomaterials Market Key Performance Indicators |
9 Netherlands Orthopedic Biomaterials Market - Opportunity Assessment |
9.1 Netherlands Orthopedic Biomaterials Market Opportunity Assessment, By Material Type, 2024 & 2031F |
9.2 Netherlands Orthopedic Biomaterials Market Opportunity Assessment, By Application, 2024 & 2031F |
9.3 Netherlands Orthopedic Biomaterials Market Opportunity Assessment, By End User, 2024 & 2031F |
9.4 Netherlands Orthopedic Biomaterials Market Opportunity Assessment, By Product Form, 2024 & 2031F |
10 Netherlands Orthopedic Biomaterials Market - Competitive Landscape |
10.1 Netherlands Orthopedic Biomaterials Market Revenue Share, By Companies, 2024 |
10.2 Netherlands Orthopedic Biomaterials Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















