Peru Catheter Market Outlook | Share, COVID-19 IMPACT, Revenue, Size, Trends, Analysis, Forecast, Value, Growth, Companies & Industry
| Product Code: ETC127073 | Publication Date: Jun 2021 | Updated Date: Jun 2025 | Product Type: Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 70 | No. of Figures: 35 | No. of Tables: 5 |
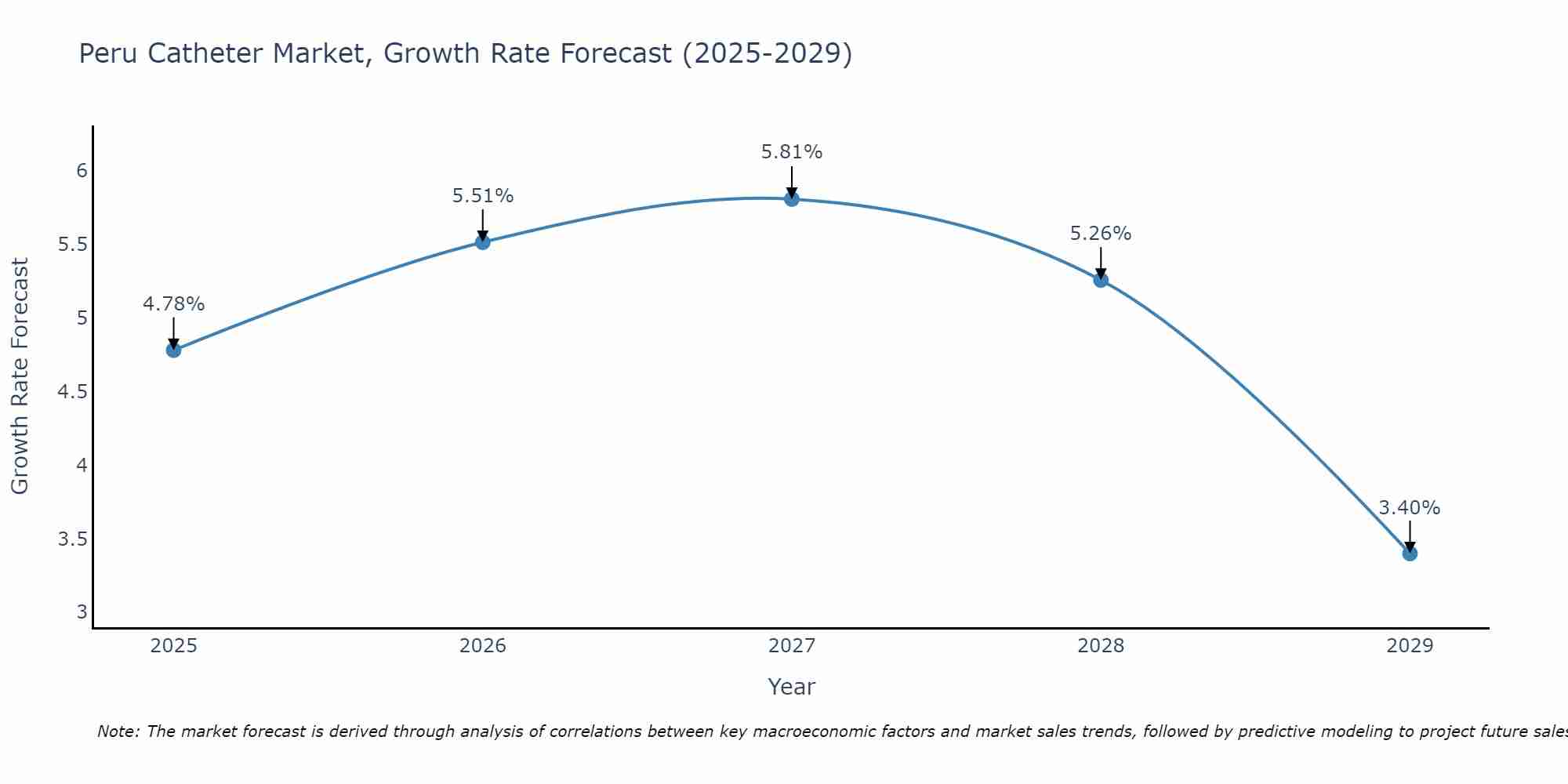
Peru Catheter Market Size Growth Rate
The Peru Catheter Market is projected to witness mixed growth rate patterns during 2025 to 2029. Starting at 4.78% in 2025, the market peaks at 5.81% in 2027, and settles at 3.40% by 2029.
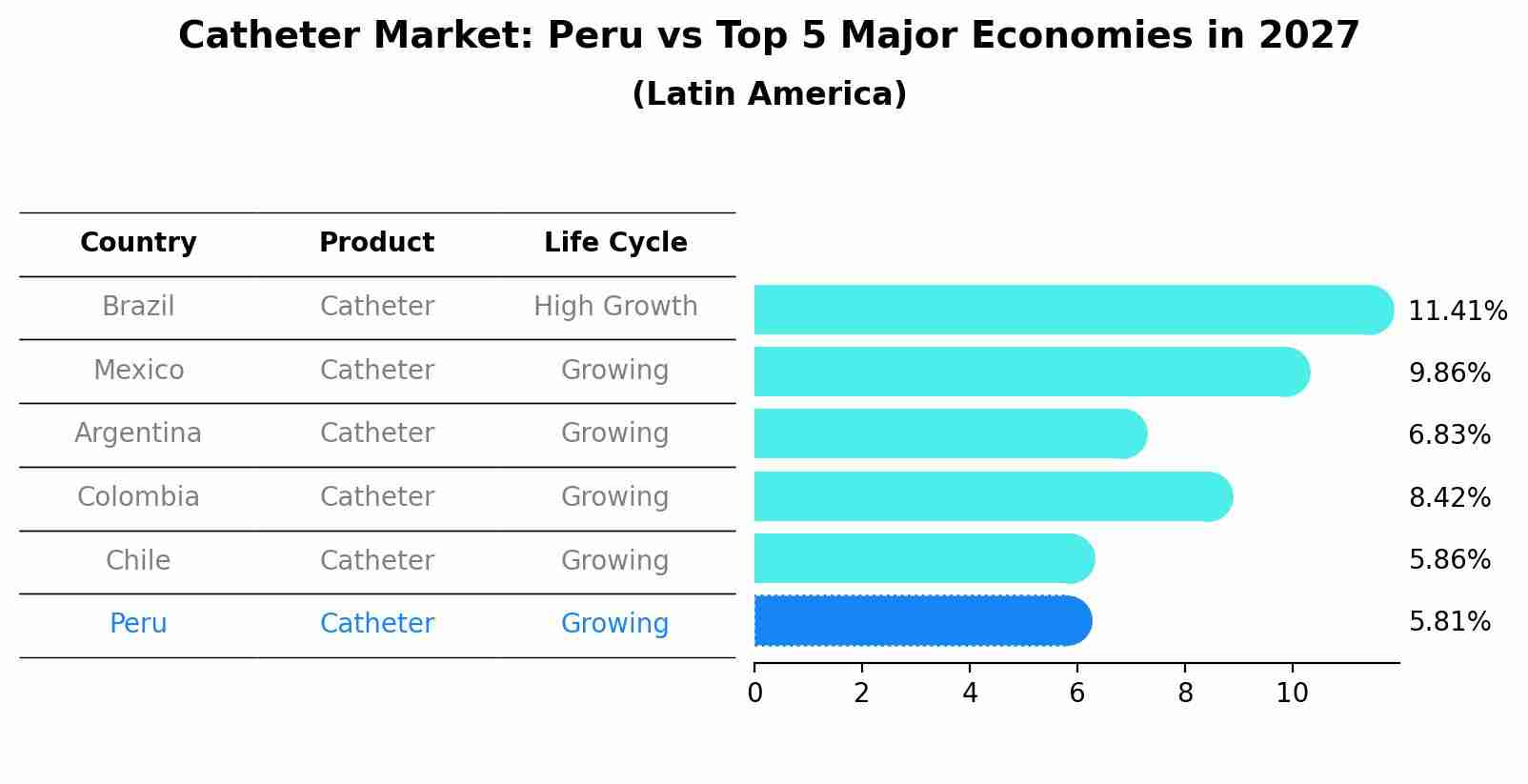
Catheter Market: Peru vs Top 5 Major Economies in 2027 (Latin America)
By 2027, the Catheter market in Peru is anticipated to reach a growth rate of 5.81%, as part of an increasingly competitive Latin America region, where Brazil remains at the forefront, supported by Mexico, Argentina, Colombia and Chile, driving innovations and market adoption across sectors.
Peru Catheter Market Overview
The Peru Catheter Market is witnessing steady growth due to increasing prevalence of cardiovascular diseases, urinary disorders, and the growing demand for minimally invasive procedures. The market is characterized by a wide range of catheter types including cardiovascular catheters, urological catheters, neurovascular catheters, and others. Key drivers of market growth include technological advancements in catheter design, rising healthcare infrastructure, and a growing elderly population. Major players operating in the Peru Catheter Market include Medtronic, Boston Scientific, Abbott, and B. Braun Melsungen AG. The market is also seeing increased adoption of advanced catheter technologies such as intravascular ultrasound and drug-coated catheters. However, regulatory challenges and limited reimbursement policies may hinder market growth in the coming years.
Peru Catheter Market Trends
The Peru Catheter Market is witnessing several key trends. One significant trend is the increasing adoption of minimally invasive procedures, driving the demand for catheters in the country. This trend is fueled by the benefits of minimally invasive techniques, such as reduced recovery times and lower risk of complications. Another emerging trend is the growing prevalence of chronic diseases, leading to a higher demand for catheters for diagnostic and therapeutic purposes. Additionally, technological advancements in catheter design and materials are improving patient comfort and overall procedural outcomes. The market is also seeing a rise in strategic collaborations and partnerships among key players to expand their product offerings and strengthen their market presence. Overall, these trends indicate a positive outlook for the Peru Catheter Market with continued growth opportunities.
Peru Catheter Market Challenges
In the Peru Catheter Market, some key challenges include intense competition among manufacturers leading to price wars and margin pressures, regulatory hurdles related to product approvals and compliance, limited healthcare infrastructure in certain regions affecting distribution and access to catheters, and a lack of awareness and training among healthcare professionals regarding advanced catheter technologies. Additionally, the market may face challenges related to currency fluctuations impacting import costs and economic instability affecting overall healthcare spending. To succeed in this market, companies need to navigate these challenges by focusing on innovation, regulatory compliance, strategic pricing strategies, and building strong partnerships with healthcare providers to ensure efficient distribution and education on catheter use.
Peru Catheter Market Investment Opportunities
In the Peru Catheter Market, there are several investment opportunities for growth and innovation. With the increasing prevalence of chronic diseases and the growing aging population in Peru, there is a rising demand for advanced catheter technologies and medical devices. Investors can look into opportunities to invest in the development and commercialization of innovative catheter products that cater to specific medical needs, such as minimally invasive procedures, improved patient comfort, and reduced risk of infection. Additionally, partnerships with local healthcare providers and distributors can help penetrate the Peruvian market more effectively. Investing in research and development for new catheter technologies and materials, as well as investing in marketing strategies to raise awareness and educate healthcare professionals about the benefits of advanced catheter solutions, can also be lucrative opportunities in the Peru Catheter Market.
Peru Catheter Market Government Policy
Government policies related to the Peru Catheter Market include regulations set by the General Directorate of Medicines, Supplies, and Drugs (DIGEMID) under the Ministry of Health. These regulations govern the registration, importation, distribution, and quality control of medical devices, including catheters, to ensure safety and efficacy. Manufacturers and distributors are required to comply with these regulations to obtain market authorization for their products. Additionally, the government has implemented measures to promote the local production of medical devices, which may impact the importation and distribution of catheters in Peru. Overall, the government policies aim to safeguard public health by ensuring that catheters meet quality standards and are used safely in healthcare settings.
Peru Catheter Market Future Outlook
The Peru Catheter Market is expected to witness steady growth in the coming years due to an increasing prevalence of chronic diseases and a growing aging population requiring medical interventions. Technological advancements in catheter design and materials are anticipated to drive market expansion, leading to the introduction of innovative products with improved patient outcomes. Additionally, rising healthcare expenditure and improving healthcare infrastructure in Peru are likely to support market growth by increasing access to advanced medical devices. However, pricing pressures and regulatory challenges may pose some limitations to market growth. Overall, the Peru Catheter Market is poised for a positive trajectory with opportunities for market players to capitalize on the growing demand for minimally invasive medical procedures.
Key Highlights of the Report:
- Peru Catheter Market Outlook
- Market Size of Peru Catheter Market, 2021
- Forecast of Peru Catheter Market, 2027
- Historical Data and Forecast of Peru Catheter Revenues & Volume for the Period 2018 - 2027
- Peru Catheter Market Trend Evolution
- Peru Catheter Market Drivers and Challenges
- Peru Catheter Price Trends
- Peru Catheter Porter's Five Forces
- Peru Catheter Industry Life Cycle
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Product Type for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Cardiovascular Catheters for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Urology Catheters for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Intravenous Catheters for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Neurovascular Catheters for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Specialty Catheters for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Distribution Channel for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Hospital Stores for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Retail Stores for the Period 2018 - 2027
- Historical Data and Forecast of Peru Catheter Market Revenues & Volume By Others for the Period 2018 - 2027
- Peru Catheter Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Distribution Channel
- Peru Catheter Top Companies Market Share
- Peru Catheter Competitive Benchmarking By Technical and Operational Parameters
- Peru Catheter Company Profiles
- Peru Catheter Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
Peru Catheter |
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Peru Catheter Market Overview |
3.1 Peru Country Macro Economic Indicators |
3.2 Peru Catheter Market Revenues & Volume, 2021 & 2027F |
3.3 Peru Catheter Market - Industry Life Cycle |
3.4 Peru Catheter Market - Porter's Five Forces |
3.5 Peru Catheter Market Revenues & Volume Share, By Product Type, 2021 & 2027F |
3.6 Peru Catheter Market Revenues & Volume Share, By Distribution Channel, 2021 & 2027F |
4 Peru Catheter Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.3 Market Restraints |
5 Peru Catheter Market Trends |
6 Peru Catheter Market, By Types |
6.1 Peru Catheter Market, By Product Type |
6.1.1 Overview and Analysis |
6.1.2 Peru Catheter Market Revenues & Volume, By Product Type, 2018 - 2027F |
6.1.3 Peru Catheter Market Revenues & Volume, By Cardiovascular Catheters, 2018 - 2027F |
6.1.4 Peru Catheter Market Revenues & Volume, By Urology Catheters, 2018 - 2027F |
6.1.5 Peru Catheter Market Revenues & Volume, By Intravenous Catheters, 2018 - 2027F |
6.1.6 Peru Catheter Market Revenues & Volume, By Neurovascular Catheters, 2018 - 2027F |
6.1.7 Peru Catheter Market Revenues & Volume, By Specialty Catheters, 2018 - 2027F |
6.2 Peru Catheter Market, By Distribution Channel |
6.2.1 Overview and Analysis |
6.2.2 Peru Catheter Market Revenues & Volume, By Hospital Stores, 2018 - 2027F |
6.2.3 Peru Catheter Market Revenues & Volume, By Retail Stores, 2018 - 2027F |
6.2.4 Peru Catheter Market Revenues & Volume, By Others, 2018 - 2027F |
7 Peru Catheter Market Import-Export Trade Statistics |
7.1 Peru Catheter Market Export to Major Countries |
7.2 Peru Catheter Market Imports from Major Countries |
8 Peru Catheter Market Key Performance Indicators |
9 Peru Catheter Market - Opportunity Assessment |
9.1 Peru Catheter Market Opportunity Assessment, By Product Type, 2021 & 2027F |
9.2 Peru Catheter Market Opportunity Assessment, By Distribution Channel, 2021 & 2027F |
10 Peru Catheter Market - Competitive Landscape |
10.1 Peru Catheter Market Revenue Share, By Companies, 2021 |
10.2 Peru Catheter Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Portugal Occupational Health & Safety Services Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Netherlands Occupational Health and Safety Services Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Belgium and Luxembourg Facility Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Russia Women Intimate Apparel Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Africa Chocolate Market (2025-2031) | Size, Share, Trends, Growth, Revenue, Analysis, Forecast, industry & Outlook
- Global Hydroxychloroquine And Chloroquine Market (2025-2031) | Industry, Trends, Size, Outlook, Growth, Value, Companies, Revenue, Analysis, Share, Forecast
- Saudi Arabia Plant Maintenance Market (2025-2031) | Industry, Size, Growth, Revenue, Value, Companies, Forecast, Analysis, Share & Trends
- Taiwan Electric Truck Market (2025-2031) | Outlook, Industry, Revenue, Size, Forecast, Growth, Analysis, Share, Companies, Value & Trends
- South Korea Electric Bus Market (2025-2031) | Outlook, Industry, Companies, Analysis, Size, Revenue, Value, Forecast, Trends, Growth & Share
- Africa Low Temperature Powder Coating Market (2025-2031) | Companies, Competition, Size, Challenges, Segmentation, Trends, Competitive, Industry, Supply, Strategy, Investment Trends, Growth, Segments, Restraints, Strategic Insights, Revenue, Share, Forecast, Drivers, Analysis, Pricing Analysis, Demand, Consumer Insights, Value, Opportunities, Outlook
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













