Philippines Smart Pulse Oximeters Market (2025-2031) | Companies, Growth, Size & Revenue, Analysis, Value, Share, Trends, Industry, Forecast, Outlook, Competitive Landscape, Segmentation
| Product Code: ETC8851783 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
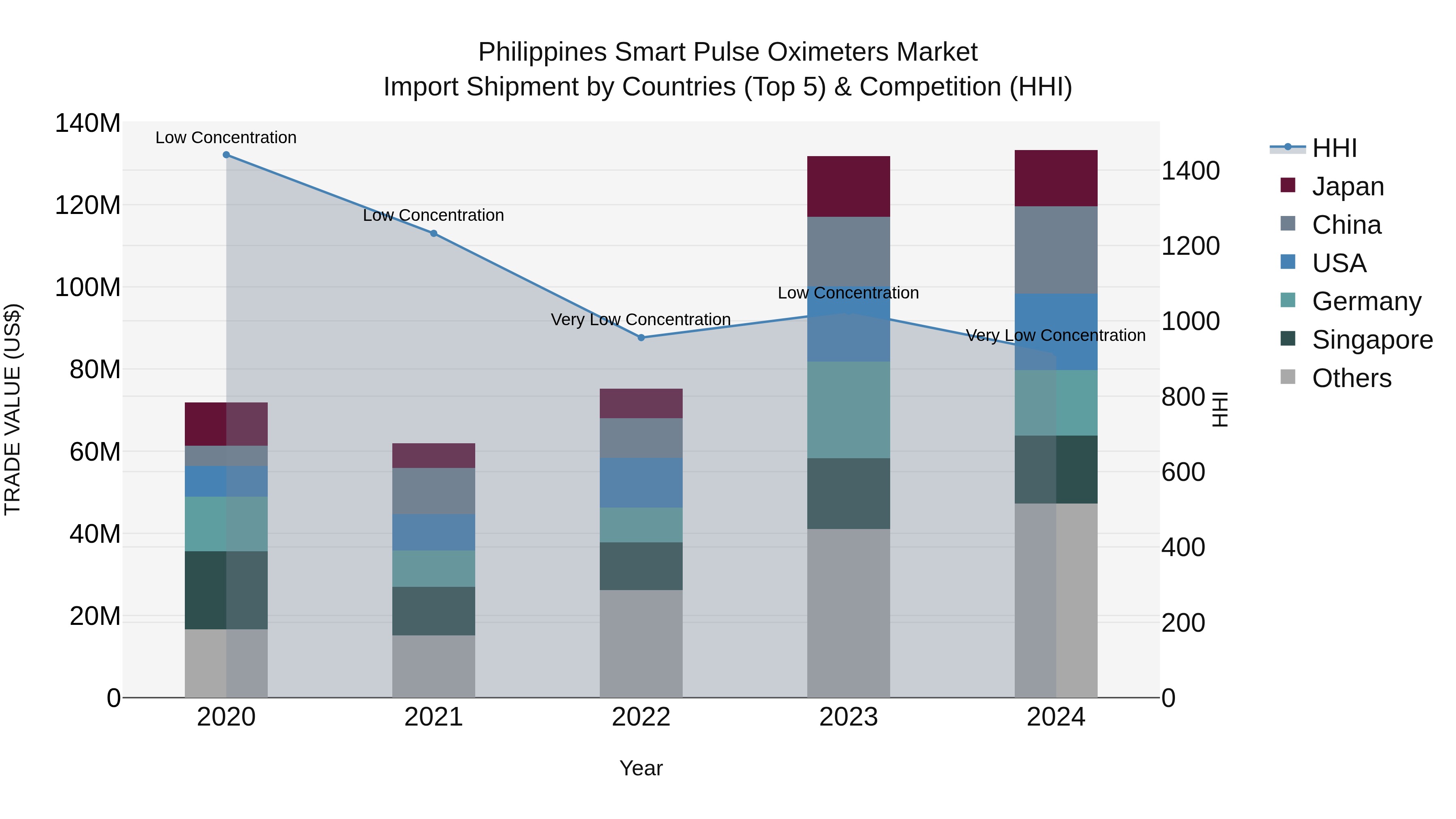
Philippines Smart Pulse Oximeters Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The Philippines is witnessing a steady rise in smart pulse oximeters import shipments, with top exporters including China, USA, Singapore, Germany, and Japan in 2024. The market shows low concentration with a declining Herfindahl-Hirschman Index (HHI) indicating a competitive landscape. A strong compound annual growth rate (CAGR) of 16.7% from 2020 to 2024 highlights the increasing demand for these medical devices. Although the growth rate slightly slowed in 2024 with a 1.13% increase from the previous year, the market remains promising for both local and international suppliers.
Philippines Smart Pulse Oximeters Market Overview
Smart pulse oximeters are increasingly used in homes and clinics for monitoring blood oxygen levels, especially in respiratory illnesses and post-COVID care. Their affordability and connectivity features make them a key tool in digital healthcare.
Trends of the market
The smart pulse oximeters market in the Philippines is gaining traction as the demand for non-invasive monitoring solutions grows, especially in response to the COVID-19 pandemic. These devices provide real-time monitoring of blood oxygen levels, making them essential for managing respiratory conditions such as COPD, asthma, and COVID-19. The increasing awareness of the importance of early detection and continuous monitoring is driving the adoption of smart pulse oximeters among healthcare providers and individuals alike. As healthcare systems become more integrated with technology, the market for these devices is expected to expand.
Challenges of the market
The Smart Pulse Oximeters market faces competition from conventional devices, market saturation post-pandemic, and accuracy concerns. While interest surged during COVID-19, demand has since declined, and consumers often prefer more affordable, basic models. Additionally, questions regarding the accuracy and calibration of some smart models reduce consumer confidence, especially among healthcare professionals.
Investment opportunities in the Market
The smart pulse oximeter market in the Philippines is poised for growth due to the increasing demand for wearable health devices. Smart pulse oximeters, which measure blood oxygen levels and heart rate, provide users with real-time data and health insights. Investment in the development of these devices, including improvements in accuracy, portability, and connectivity to health apps, presents an opportunity in the growing healthcare technology sector.
Government Policy of the market
DOH has issued policy circulars supporting remote monitoring tools like smart pulse oximeters, particularly for COVID-19 home care. The FDA has also adjusted regulatory pathways for rapid approval, and some LGUs have incorporated these devices into local public health protocols, reinforcing their use in preventive healthcare.
Key Highlights of the Report:
- Philippines Smart Pulse Oximeters Market Outlook
- Market Size of Philippines Smart Pulse Oximeters Market, 2024
- Forecast of Philippines Smart Pulse Oximeters Market, 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Revenues & Volume for the Period 2021- 2031
- Philippines Smart Pulse Oximeters Market Trend Evolution
- Philippines Smart Pulse Oximeters Market Drivers and Challenges
- Philippines Smart Pulse Oximeters Price Trends
- Philippines Smart Pulse Oximeters Porter's Five Forces
- Philippines Smart Pulse Oximeters Industry Life Cycle
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Product Type for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Finger Pulse Oximeter for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Wrist Pulse Oximeter for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Others for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Distribution Channel for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Retail Stores for the Period 2021- 2031
- Historical Data and Forecast of Philippines Smart Pulse Oximeters Market Revenues & Volume By Online Stores for the Period 2021- 2031
- Philippines Smart Pulse Oximeters Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Distribution Channel
- Philippines Smart Pulse Oximeters Top Companies Market Share
- Philippines Smart Pulse Oximeters Competitive Benchmarking By Technical and Operational Parameters
- Philippines Smart Pulse Oximeters Company Profiles
- Philippines Smart Pulse Oximeters Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
Export potential assessment - trade Analytics for 2030
Export potential enables firms to identify high-growth global markets with greater confidence by combining advanced trade intelligence with a structured quantitative methodology. The framework analyzes emerging demand trends and country-level import patterns while integrating macroeconomic and trade datasets such as GDP and population forecasts, bilateral import–export flows, tariff structures, elasticity differentials between developed and developing economies, geographic distance, and import demand projections. Using weighted trade values from 2020–2024 as the base period to project country-to-country export potential for 2030, these inputs are operationalized through calculated drivers such as gravity model parameters, tariff impact factors, and projected GDP per-capita growth. Through an analysis of hidden potentials, demand hotspots, and market conditions that are most favorable to success, this method enables firms to focus on target countries, maximize returns, and global expansion with data, backed by accuracy.
By factoring in the projected importer demand gap that is currently unmet and could be potential opportunity, it identifies the potential for the Exporter (Country) among 190 countries, against the general trade analysis, which identifies the biggest importer or exporter.
To discover high-growth global markets and optimize your business strategy:
Click Here- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- India Switchgear Market Outlook (2026 - 2032) | Size, Share, Trends, Growth, Revenue, Forecast, Analysis, Value, Outlook
- Pakistan Contraceptive Implants Market (2025-2031) | Demand, Growth, Size, Share, Industry, Pricing Analysis, Competitive, Strategic Insights, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Companies, Challenges
- Sri Lanka Packaging Market (2026-2032) | Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints
- India Kids Watches Market (2026-2032) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Saudi Arabia Core Assurance Service Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Romania Uninterruptible Power Supply (UPS) Market (2026-2032) | Industry, Analysis, Revenue, Size, Forecast, Outlook, Value, Trends, Share, Growth & Companies
- Saudi Arabia Car Window Tinting Film, Paint Protection Film (PPF), and Ceramic Coating Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















