Slovakia Trauma Fixation Devices Market (2025-2031) | Outlook, Size & Revenue, Competitive Landscape, Forecast, Trends, Growth, Value, Companies, Industry, Share, Segmentation, Analysis
| Product Code: ETC9307876 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
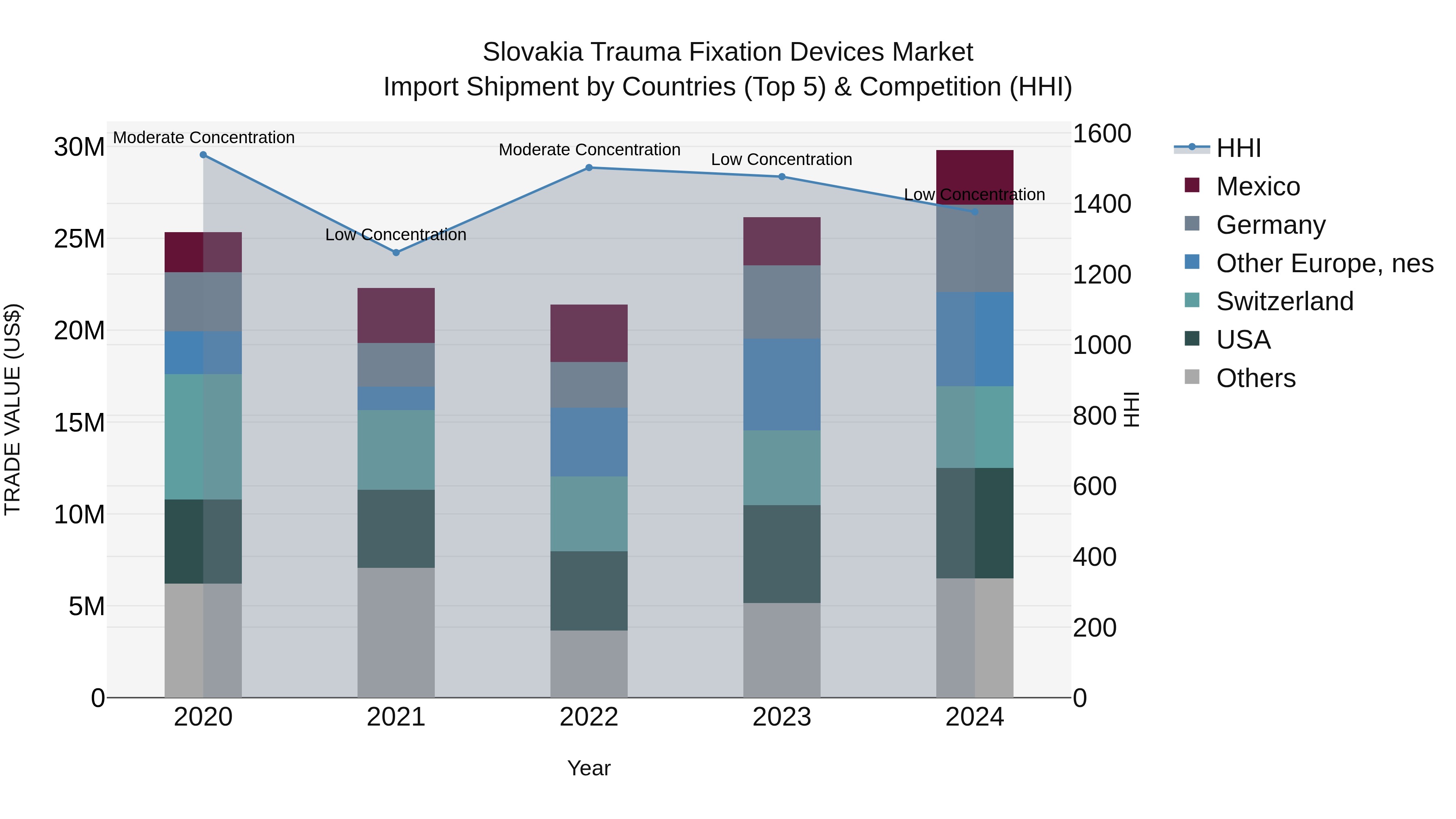
Slovakia Trauma Fixation Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
Slovakia`s import of trauma fixation devices in 2024 saw a diverse range of top exporting countries, including the USA, Germany, Switzerland, and Mexico. The Market Top 5 Importing Countries and Market Competition (HHI) Analysis remained relatively unconcentrated with a low Herfindahl-Hirschman Index (HHI). The compound annual growth rate (CAGR) from 2020 to 2024 was a steady 4.14%, indicating a stable Market Top 5 Importing Countries and Market Competition (HHI) Analysis expansion. Notably, the growth rate spiked in 2024 with a 13.99% increase from the previous year, showcasing a promising upward trend in the importation of trauma fixation devices in Slovakia.
Slovakia Trauma Fixation Devices Market Overview
The Slovakia Trauma Fixation Devices Market is experiencing steady growth driven by an increasing incidence of musculoskeletal injuries and road accidents. Key players in the market are focusing on developing innovative and advanced trauma fixation devices to cater to the rising demand for effective treatment options. The market is characterized by a competitive landscape with companies such as DePuy Synthes, Stryker Corporation, and Smith & Nephew PLC holding significant market shares. Surgeons and healthcare professionals in Slovakia are increasingly adopting minimally invasive techniques for trauma fixation procedures, leading to a higher demand for specialized devices. The market is expected to witness further growth with advancements in technology, rising healthcare infrastructure, and increasing awareness about the benefits of trauma fixation devices among patients and healthcare providers.
Slovakia Trauma Fixation Devices Market Trends and Opportunities
The Slovakia Trauma Fixation Devices Market is witnessing a growing demand for minimally invasive surgical procedures, leading to an increasing adoption of advanced trauma fixation devices. Key trends include the development of biodegradable materials for implants, technological advancements in implant design for better patient outcomes, and the rise in geriatric population contributing to the higher incidence of trauma cases. Opportunities in the market lie in the expansion of product portfolios by key players, strategic partnerships for distribution networks, and the increasing focus on customized implants for better patient care. Additionally, the emphasis on research and development to introduce innovative solutions tailored to specific trauma cases presents avenues for market growth and differentiation.
Slovakia Trauma Fixation Devices Market Challenges
In the Slovakia Trauma Fixation Devices Market, some challenges include intense competition from established market players, price sensitivity among hospitals and healthcare facilities, regulatory hurdles in terms of product registration and approval processes, and the increasing demand for technologically advanced products. Additionally, the market may face challenges related to the limited availability of skilled healthcare professionals specialized in trauma surgeries, which can impact the adoption and usage of trauma fixation devices. Moreover, economic factors and budget constraints within the healthcare sector may also pose challenges for market growth and expansion in Slovakia. Overall, navigating these challenges requires companies to innovate, streamline their operations, and build strong relationships with key stakeholders in the healthcare industry.
Slovakia Trauma Fixation Devices Market Drivers
The Slovakia Trauma Fixation Devices Market is primarily driven by several factors, including the increasing incidence of traumatic injuries, road accidents, and sports-related injuries requiring surgical intervention. Additionally, the rising geriatric population prone to fractures and the growing demand for minimally invasive surgeries are contributing to the market growth. Advances in technology leading to the development of innovative trauma fixation devices, along with an expanding healthcare infrastructure and increasing healthcare expenditure, are also fueling market expansion. Moreover, the surging awareness about the benefits of timely and effective treatment for traumatic injuries among both healthcare providers and patients is further boosting the demand for trauma fixation devices in Slovakia.
Slovakia Trauma Fixation Devices Market Government Policies
The Slovakia Trauma Fixation Devices Market is subject to government regulations aimed at ensuring the safety and efficacy of these medical devices. These regulations encompass requirements for product registration, quality control, and post-market surveillance to monitor device performance and address any safety concerns. The Slovak regulatory authority, the State Institute for Drug Control (SUKL), oversees the approval and monitoring of trauma fixation devices in the market. Manufacturers must comply with the European Union`s Medical Device Regulation (MDR) to market their products in Slovakia, which includes rigorous clinical evaluation and conformity assessment processes. Adherence to these government policies is crucial for companies operating in the Slovakia Trauma Fixation Devices Market to maintain compliance and market access.
Slovakia Trauma Fixation Devices Market Future Outlook
The Slovakia Trauma Fixation Devices Market is anticipated to witness steady growth in the coming years due to several factors such as the increasing incidence of traumatic injuries, advancements in medical technology, and a growing geriatric population. The demand for trauma fixation devices is expected to rise as healthcare infrastructure continues to improve in Slovakia, leading to better access to advanced medical treatments. Additionally, the rising awareness about the importance of timely and effective trauma care among both healthcare providers and patients is likely to drive market growth. Market players are also focusing on developing innovative and cost-effective solutions to meet the evolving needs of healthcare professionals, further contributing to the market`s expansion. Overall, the Slovakia Trauma Fixation Devices Market is poised for growth in the foreseeable future.
Key Highlights of the Report:
- Slovakia Trauma Fixation Devices Market Outlook
- Market Size of Slovakia Trauma Fixation Devices Market, 2024
- Forecast of Slovakia Trauma Fixation Devices Market, 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Revenues & Volume for the Period 2021- 2031
- Slovakia Trauma Fixation Devices Market Trend Evolution
- Slovakia Trauma Fixation Devices Market Drivers and Challenges
- Slovakia Trauma Fixation Devices Price Trends
- Slovakia Trauma Fixation Devices Porter's Five Forces
- Slovakia Trauma Fixation Devices Industry Life Cycle
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By Type for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By Internal Fixation Devices for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By External Fixation Devices for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By Application for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By ASCs for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By Physicians Offices for the Period 2021- 2031
- Historical Data and Forecast of Slovakia Trauma Fixation Devices Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Slovakia Trauma Fixation Devices Import Export Trade Statistics
- Market Opportunity Assessment By Type
- Market Opportunity Assessment By Application
- Slovakia Trauma Fixation Devices Top Companies Market Share
- Slovakia Trauma Fixation Devices Competitive Benchmarking By Technical and Operational Parameters
- Slovakia Trauma Fixation Devices Company Profiles
- Slovakia Trauma Fixation Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 Slovakia Trauma Fixation Devices Market Overview |
3.1 Slovakia Country Macro Economic Indicators |
3.2 Slovakia Trauma Fixation Devices Market Revenues & Volume, 2021 & 2031F |
3.3 Slovakia Trauma Fixation Devices Market - Industry Life Cycle |
3.4 Slovakia Trauma Fixation Devices Market - Porter's Five Forces |
3.5 Slovakia Trauma Fixation Devices Market Revenues & Volume Share, By Type, 2021 & 2031F |
3.6 Slovakia Trauma Fixation Devices Market Revenues & Volume Share, By Application, 2021 & 2031F |
4 Slovakia Trauma Fixation Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.3 Market Restraints |
5 Slovakia Trauma Fixation Devices Market Trends |
6 Slovakia Trauma Fixation Devices Market, By Types |
6.1 Slovakia Trauma Fixation Devices Market, By Type |
6.1.1 Overview and Analysis |
6.1.2 Slovakia Trauma Fixation Devices Market Revenues & Volume, By Type, 2021- 2031F |
6.1.3 Slovakia Trauma Fixation Devices Market Revenues & Volume, By Internal Fixation Devices, 2021- 2031F |
6.1.4 Slovakia Trauma Fixation Devices Market Revenues & Volume, By External Fixation Devices, 2021- 2031F |
6.2 Slovakia Trauma Fixation Devices Market, By Application |
6.2.1 Overview and Analysis |
6.2.2 Slovakia Trauma Fixation Devices Market Revenues & Volume, By ASCs, 2021- 2031F |
6.2.3 Slovakia Trauma Fixation Devices Market Revenues & Volume, By Physicians Offices, 2021- 2031F |
6.2.4 Slovakia Trauma Fixation Devices Market Revenues & Volume, By Hospitals, 2021- 2031F |
7 Slovakia Trauma Fixation Devices Market Import-Export Trade Statistics |
7.1 Slovakia Trauma Fixation Devices Market Export to Major Countries |
7.2 Slovakia Trauma Fixation Devices Market Imports from Major Countries |
8 Slovakia Trauma Fixation Devices Market Key Performance Indicators |
9 Slovakia Trauma Fixation Devices Market - Opportunity Assessment |
9.1 Slovakia Trauma Fixation Devices Market Opportunity Assessment, By Type, 2021 & 2031F |
9.2 Slovakia Trauma Fixation Devices Market Opportunity Assessment, By Application, 2021 & 2031F |
10 Slovakia Trauma Fixation Devices Market - Competitive Landscape |
10.1 Slovakia Trauma Fixation Devices Market Revenue Share, By Companies, 2024 |
10.2 Slovakia Trauma Fixation Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
Export potential assessment - trade Analytics for 2030
Export potential enables firms to identify high-growth global markets with greater confidence by combining advanced trade intelligence with a structured quantitative methodology. The framework analyzes emerging demand trends and country-level import patterns while integrating macroeconomic and trade datasets such as GDP and population forecasts, bilateral import–export flows, tariff structures, elasticity differentials between developed and developing economies, geographic distance, and import demand projections. Using weighted trade values from 2020–2024 as the base period to project country-to-country export potential for 2030, these inputs are operationalized through calculated drivers such as gravity model parameters, tariff impact factors, and projected GDP per-capita growth. Through an analysis of hidden potentials, demand hotspots, and market conditions that are most favorable to success, this method enables firms to focus on target countries, maximize returns, and global expansion with data, backed by accuracy.
By factoring in the projected importer demand gap that is currently unmet and could be potential opportunity, it identifies the potential for the Exporter (Country) among 190 countries, against the general trade analysis, which identifies the biggest importer or exporter.
To discover high-growth global markets and optimize your business strategy:
Click Here- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- India Switchgear Market Outlook (2026 - 2032) | Size, Share, Trends, Growth, Revenue, Forecast, Analysis, Value, Outlook
- Pakistan Contraceptive Implants Market (2025-2031) | Demand, Growth, Size, Share, Industry, Pricing Analysis, Competitive, Strategic Insights, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Companies, Challenges
- Sri Lanka Packaging Market (2026-2032) | Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges, Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints
- India Kids Watches Market (2026-2032) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Saudi Arabia Core Assurance Service Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Romania Uninterruptible Power Supply (UPS) Market (2026-2032) | Industry, Analysis, Revenue, Size, Forecast, Outlook, Value, Trends, Share, Growth & Companies
- Saudi Arabia Car Window Tinting Film, Paint Protection Film (PPF), and Ceramic Coating Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















