United States (US) Hemodynamic Monitoring Devices Market (2025-2031) | Competitive Landscape, Share, Trends, Segmentation, Industry, Size & Revenue, Growth, Outlook, Value, Forecast, Companies, Analysis
| Product Code: ETC9967599 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
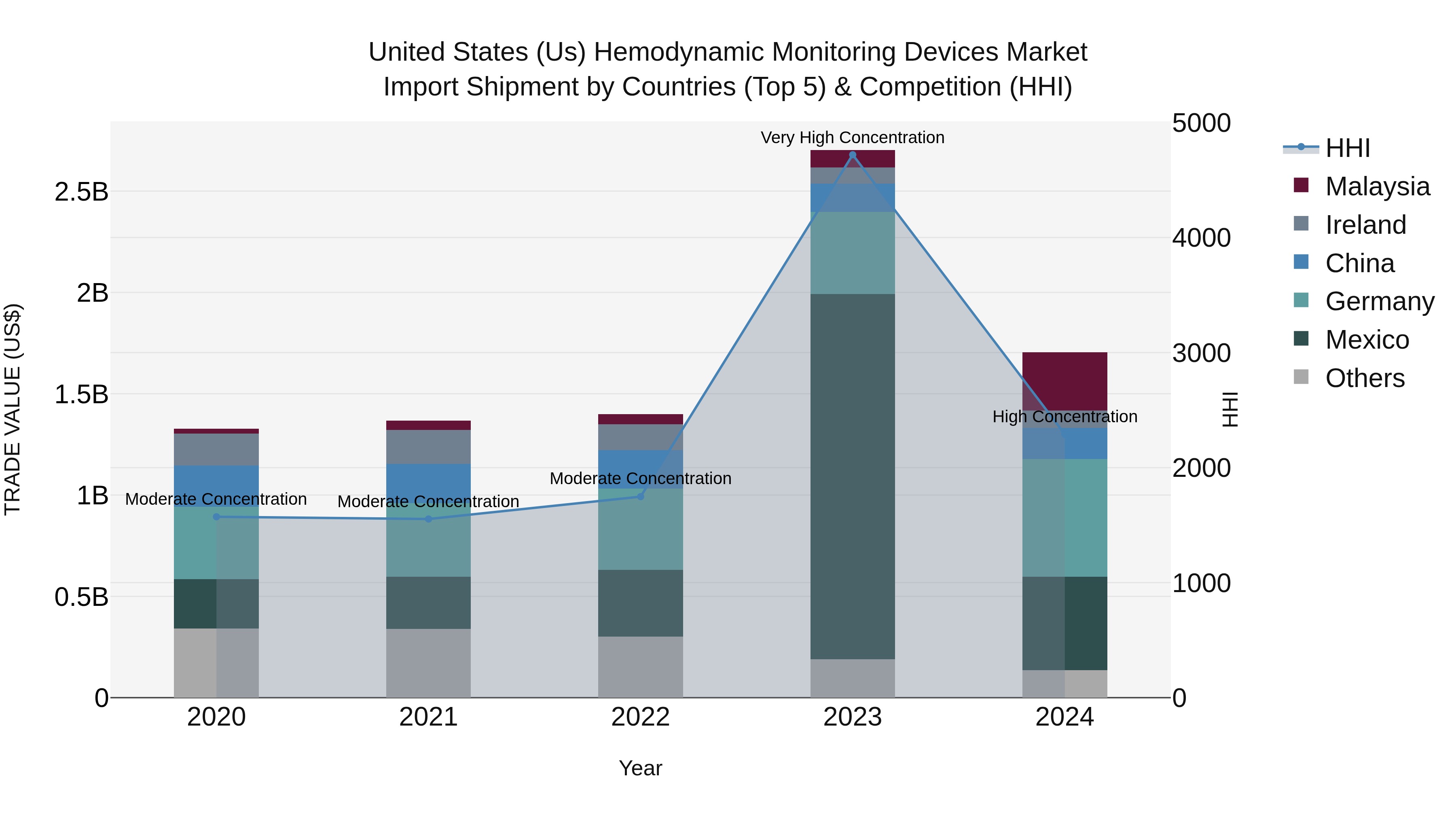
United States (US) Hemodynamic Monitoring Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
The hemodynamic monitoring devices import shipments to the United States in 2024 saw a shift in the top exporting countries, with Germany, Mexico, Malaysia, China, and Ireland leading the way. The HHI concentration remained high, indicating market dominance, which could impact competition and pricing. The CAGR from 2020 to 2024 shows steady growth at 6.47%, although there was a notable decline in the growth rate from 2023 to 2024 at -36.9%. This fluctuation may suggest evolving market dynamics and potential challenges ahead in the hemodynamic monitoring devices sector.
United States (US) Hemodynamic Monitoring Devices Market Synopsis
The United States Hemodynamic Monitoring Devices Market is a rapidly growing sector within the medical device industry, driven by factors such as the increasing prevalence of cardiovascular diseases, technological advancements in monitoring devices, and the rising demand for minimally invasive procedures. These devices play a crucial role in monitoring the hemodynamic status of patients in critical care settings, providing real-time data on parameters such as blood pressure, cardiac output, and oxygen saturation. Key players in the market include Edwards Lifesciences Corporation, ICU Medical Inc., and Cheetah Medical Inc. The market is characterized by a high level of competition, with companies focusing on product innovation, strategic collaborations, and geographic expansion to gain a competitive edge. With the growing emphasis on patient safety and the need for accurate and timely monitoring, the US Hemodynamic Monitoring Devices Market is expected to continue its growth trajectory in the coming years.
United States (US) Hemodynamic Monitoring Devices Market Trends
The US Hemodynamic Monitoring Devices Market is experiencing a shift towards minimally invasive and non-invasive monitoring solutions, driven by the increasing demand for improved patient outcomes and reduced healthcare costs. Technologies such as wireless monitoring systems and wearable devices are gaining traction due to their convenience and ability to provide real-time data. Additionally, the rising prevalence of cardiovascular diseases and the growing aging population are fueling the demand for hemodynamic monitoring devices in the US. There is also a focus on integrating advanced analytics and artificial intelligence capabilities into these devices to enhance diagnostic accuracy and clinical decision-making. Overall, the market presents opportunities for innovation, partnerships with healthcare providers, and expansion into home healthcare settings to meet the evolving needs of patients and healthcare systems.
United States (US) Hemodynamic Monitoring Devices Market Challenges
In the US Hemodynamic Monitoring Devices Market, challenges include rapid technological advancements leading to the need for continuous innovation to stay competitive, stringent regulatory requirements that impact product development and market entry, increasing healthcare costs putting pressure on hospitals to justify investments in new devices, and the need for healthcare professionals to be trained in using these complex monitoring systems effectively. Additionally, the market is highly fragmented with numerous players offering similar products, leading to intense competition and pricing pressures. Moreover, the COVID-19 pandemic has further strained healthcare resources, impacting the adoption and utilization of hemodynamic monitoring devices. Overall, navigating these challenges requires companies to invest in research and development, regulatory compliance, market education, and strategic pricing strategies to succeed in the US market.
United States (US) Hemodynamic Monitoring Devices Market Investment Opportunities
The United States Hemodynamic Monitoring Devices Market is primarily driven by factors such as the rising prevalence of cardiovascular diseases, increasing geriatric population, and the growing adoption of minimally invasive procedures. Technological advancements in hemodynamic monitoring devices, such as wireless and wearable options, are also contributing to market growth. Moreover, the emphasis on early detection and management of critical conditions like sepsis and heart failure is fueling the demand for hemodynamic monitoring devices in the US healthcare sector. Additionally, the expanding healthcare infrastructure and investments in the development of innovative monitoring solutions are expected to further propel market growth in the coming years.
United States (US) Hemodynamic Monitoring Devices Market Government Polices
The US government regulates hemodynamic monitoring devices through the Food and Drug Administration (FDA), which oversees the approval and safety of these medical devices. Companies must comply with FDA regulations to ensure the devices are safe and effective for patient use. In addition, reimbursement policies from government healthcare programs such as Medicare and Medicaid play a crucial role in shaping the market landscape for hemodynamic monitoring devices, as they determine the financial incentives for healthcare providers to adopt these technologies. The Affordable Care Act (ACA) also impacts the market by focusing on improving healthcare quality and patient outcomes, driving the demand for advanced monitoring devices that can help in achieving these goals. Overall, government policies in the US create a regulatory framework and reimbursement environment that influence the adoption and utilization of hemodynamic monitoring devices in the healthcare sector.
United States (US) Hemodynamic Monitoring Devices Market Future Outlook
The United States Hemodynamic Monitoring Devices Market is expected to witness steady growth in the coming years due to the increasing prevalence of cardiovascular diseases, the aging population, and the rising demand for minimally invasive procedures. Technological advancements such as wireless monitoring systems and non-invasive monitoring devices are likely to drive market expansion. Additionally, the growing adoption of hemodynamic monitoring in critical care settings and operating rooms will further contribute to market growth. The market is also expected to benefit from the emphasis on value-based healthcare and the focus on improving patient outcomes. However, pricing pressure and reimbursement challenges may pose some limitations to market growth. Overall, the US Hemodynamic Monitoring Devices Market is anticipated to show promising growth opportunities in the foreseeable future.
Key Highlights of the Report:
- United States (US) Hemodynamic Monitoring Devices Market Outlook
- Market Size of United States (US) Hemodynamic Monitoring Devices Market, 2024
- Forecast of United States (US) Hemodynamic Monitoring Devices Market, 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Revenues & Volume for the Period 2021- 2031
- United States (US) Hemodynamic Monitoring Devices Market Trend Evolution
- United States (US) Hemodynamic Monitoring Devices Market Drivers and Challenges
- United States (US) Hemodynamic Monitoring Devices Price Trends
- United States (US) Hemodynamic Monitoring Devices Porter's Five Forces
- United States (US) Hemodynamic Monitoring Devices Industry Life Cycle
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By System Type for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Invasive for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Minimally Invasive for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Non-Invasive for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Disposables for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Monitors for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By End Use for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Catheterization Labs for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume By Others for the Period 2021- 2031
- United States (US) Hemodynamic Monitoring Devices Import Export Trade Statistics
- Market Opportunity Assessment By System Type
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By End Use
- United States (US) Hemodynamic Monitoring Devices Top Companies Market Share
- United States (US) Hemodynamic Monitoring Devices Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Hemodynamic Monitoring Devices Company Profiles
- United States (US) Hemodynamic Monitoring Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) Hemodynamic Monitoring Devices Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) Hemodynamic Monitoring Devices Market - Industry Life Cycle |
3.4 United States (US) Hemodynamic Monitoring Devices Market - Porter's Five Forces |
3.5 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume Share, By System Type, 2021 & 2031F |
3.6 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume Share, By Product, 2021 & 2031F |
3.7 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume Share, By End Use, 2021 & 2031F |
4 United States (US) Hemodynamic Monitoring Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of cardiovascular diseases in the US |
4.2.2 Growing geriatric population requiring hemodynamic monitoring |
4.2.3 Technological advancements leading to the development of more efficient monitoring devices |
4.3 Market Restraints |
4.3.1 High cost associated with hemodynamic monitoring devices |
4.3.2 Lack of skilled healthcare professionals for accurate monitoring and interpretation |
4.3.3 Stringent regulatory requirements for approval of monitoring devices |
5 United States (US) Hemodynamic Monitoring Devices Market Trends |
6 United States (US) Hemodynamic Monitoring Devices Market, By Types |
6.1 United States (US) Hemodynamic Monitoring Devices Market, By System Type |
6.1.1 Overview and Analysis |
6.1.2 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By System Type, 2021- 2031F |
6.1.3 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Invasive, 2021- 2031F |
6.1.4 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Minimally Invasive, 2021- 2031F |
6.1.5 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Non-Invasive, 2021- 2031F |
6.2 United States (US) Hemodynamic Monitoring Devices Market, By Product |
6.2.1 Overview and Analysis |
6.2.2 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Disposables, 2021- 2031F |
6.2.3 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Monitors, 2021- 2031F |
6.3 United States (US) Hemodynamic Monitoring Devices Market, By End Use |
6.3.1 Overview and Analysis |
6.3.2 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.3.3 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Catheterization Labs, 2021- 2031F |
6.3.4 United States (US) Hemodynamic Monitoring Devices Market Revenues & Volume, By Others, 2021- 2031F |
7 United States (US) Hemodynamic Monitoring Devices Market Import-Export Trade Statistics |
7.1 United States (US) Hemodynamic Monitoring Devices Market Export to Major Countries |
7.2 United States (US) Hemodynamic Monitoring Devices Market Imports from Major Countries |
8 United States (US) Hemodynamic Monitoring Devices Market Key Performance Indicators |
8.1 Adoption rate of hemodynamic monitoring devices in US hospitals |
8.2 Rate of integration of hemodynamic monitoring devices with electronic health records systems |
8.3 Number of research studies and clinical trials utilizing hemodynamic monitoring devices in the US |
9 United States (US) Hemodynamic Monitoring Devices Market - Opportunity Assessment |
9.1 United States (US) Hemodynamic Monitoring Devices Market Opportunity Assessment, By System Type, 2021 & 2031F |
9.2 United States (US) Hemodynamic Monitoring Devices Market Opportunity Assessment, By Product, 2021 & 2031F |
9.3 United States (US) Hemodynamic Monitoring Devices Market Opportunity Assessment, By End Use, 2021 & 2031F |
10 United States (US) Hemodynamic Monitoring Devices Market - Competitive Landscape |
10.1 United States (US) Hemodynamic Monitoring Devices Market Revenue Share, By Companies, 2024 |
10.2 United States (US) Hemodynamic Monitoring Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















