Peru Urodynamic Equipment Market (2025-2031) Outlook | Trends, Share, Industry, Companies, Size, Analysis, Growth, Forecast, Revenue & Value
| Product Code: ETC370146 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Shubham Padhi | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Peru Urodynamic Equipment Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, Peru saw steady import shipments of urodynamic equipment from top exporting countries including USA, China, Germany, Japan, and Mexico. With a low Herfindahl-Hirschman Index (HHI) indicating low market concentration, the market remains competitive. Despite a slight decline in the compound annual growth rate (CAGR) from 2020 to 2024 at -2.22%, there was a notable growth rate of 14.11% from 2023 to 2024. This suggests a potential resurgence in demand for urodynamic equipment in Peru, driven by advancements in technology and healthcare infrastructure.
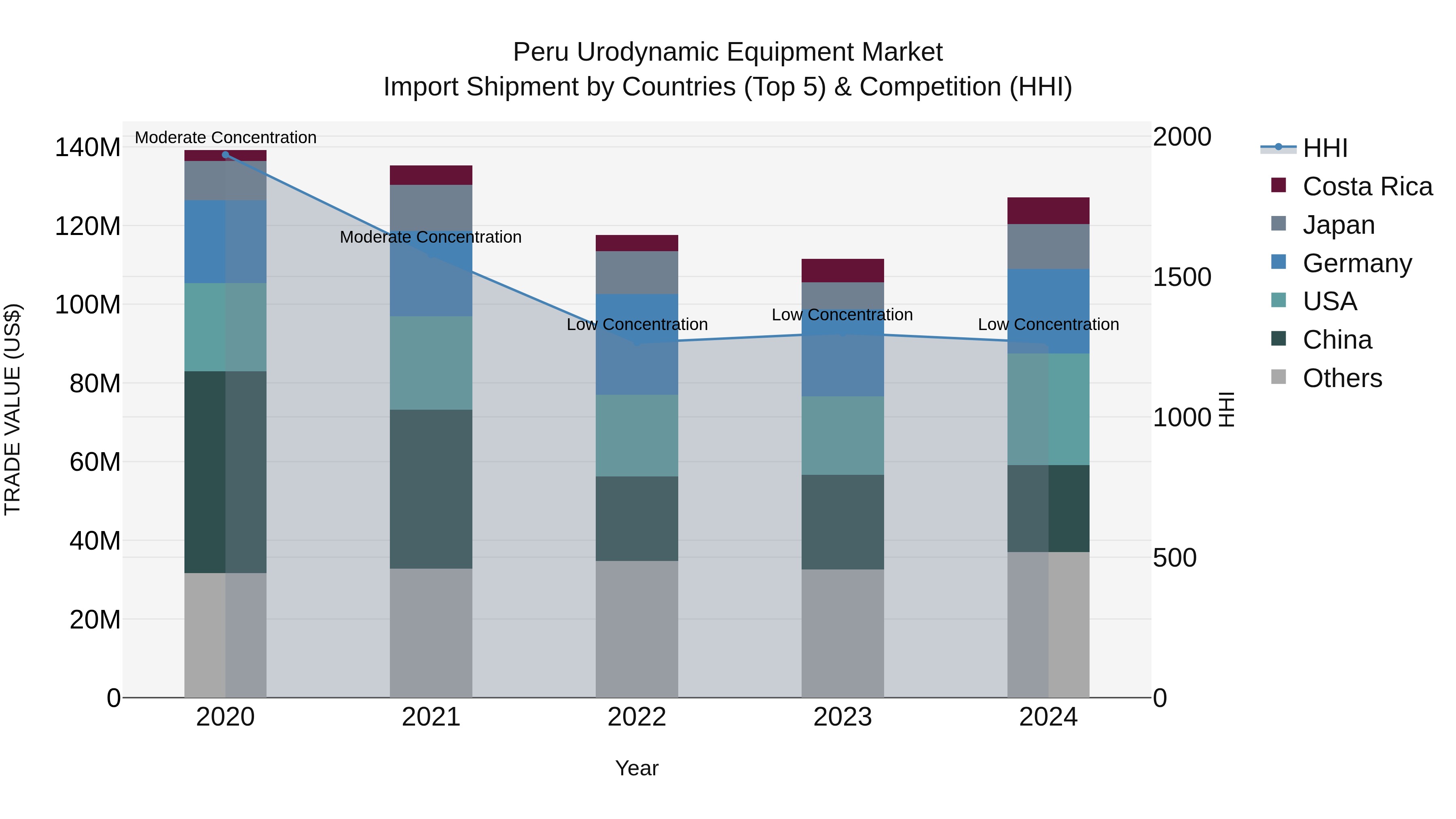
Peru Urodynamic Equipment Market Synopsis
The Peru Urodynamic Equipment Market is experiencing steady growth driven by factors such as increasing prevalence of urological disorders, growing awareness about the importance of early diagnosis, and advancements in healthcare infrastructure. The market consists of a range of urodynamic equipment including uroflowmetry systems, cystometers, electromyographs, video urodynamic systems, and pressure flow studies equipment. Key players in the market are focusing on product innovation, technological advancements, and strategic collaborations to strengthen their market presence. Additionally, rising healthcare expenditure, improving reimbursement policies, and growing elderly population are further contributing to the market growth. The market is expected to continue its expansion in the coming years, with a greater emphasis on providing accurate diagnosis and treatment for urological conditions.
Peru Urodynamic Equipment Market Trends
The Peru Urodynamic Equipment Market is witnessing several key trends. Firstly, there is a growing emphasis on the integration of advanced technologies such as wireless connectivity and data analytics in urodynamic equipment to enhance efficiency and accuracy of diagnosis. Secondly, there is an increasing demand for portable and user-friendly urodynamic devices that can be used in outpatient settings, reflecting a shift towards more convenient and accessible healthcare services. Additionally, there is a rising awareness about the importance of early detection and management of urological disorders, driving the adoption of urodynamic equipment for diagnostic purposes. Overall, the market is experiencing a trend towards innovation, portability, and patient-centric solutions to meet the evolving needs of healthcare providers and patients in Peru.
Peru Urodynamic Equipment Market Challenges
In the Peru urodynamic equipment market, several challenges are encountered. Limited awareness and knowledge about urodynamic testing among healthcare professionals and patients hinder the adoption of these diagnostic tools. Additionally, the high cost associated with urodynamic equipment and the lack of reimbursement policies in place pose financial challenges for healthcare providers and patients. Furthermore, the relatively small market size and competition from alternative diagnostic methods like ultrasound and MRI restrict the growth potential of the urodynamic equipment market in Peru. Overcoming these challenges requires targeted educational initiatives to raise awareness, strategic pricing strategies to make the equipment more accessible, and collaborations with healthcare institutions to promote the benefits of urodynamic testing for accurate diagnosis and treatment of urological conditions.
Peru Urodynamic Equipment Market Investment Opportunities
The Peru urodynamic equipment market presents promising investment opportunities due to the increasing prevalence of urological disorders and the growing demand for advanced diagnostic and treatment solutions. With a rising geriatric population and improving healthcare infrastructure in the country, there is a growing need for urodynamic equipment such as uroflowmetry systems, cystometrography equipment, and EMG systems. Investing in this market offers potential for growth, especially as healthcare providers in Peru strive to enhance their capabilities in diagnosing and managing urological conditions. Additionally, government initiatives to improve healthcare access and affordability further support the expansion of the urodynamic equipment market in Peru. Overall, investors can capitalize on the market`s growth potential by tapping into the increasing demand for innovative urodynamic technologies in the country.
Jordan Agar Market Government Policies
Government policies in Peru related to the Urodynamic Equipment Market are primarily focused on ensuring the quality and safety of medical devices. The General Health Law in Peru mandates that medical devices, including urodynamic equipment, must meet certain regulatory standards to be imported, distributed, and used in healthcare facilities. The National Institute of Health is responsible for overseeing the registration and approval process of medical devices, ensuring they comply with technical and safety requirements. Additionally, the Ministry of Health in Peru regularly updates regulations and guidelines to enhance the quality of healthcare services and protect patient safety, which indirectly influences the urodynamic equipment market by promoting the use of advanced and reliable technology in the country`s healthcare system.
Peru Urodynamic Equipment Market Future Outlook
The Peru Urodynamic Equipment Market is poised for steady growth in the coming years due to increasing awareness about urological disorders, rising prevalence of urinary incontinence, and advancements in healthcare infrastructure. The market is expected to be driven by a growing elderly population, rising healthcare expenditure, and the adoption of advanced diagnostic technologies. Additionally, the demand for minimally invasive procedures and the development of innovative urodynamic equipment are likely to further fuel market growth. Strategic collaborations between key players, healthcare providers, and government initiatives aimed at improving urological healthcare services are anticipated to propel the market forward. However, challenges such as high costs associated with urodynamic equipment and the limited availability of skilled healthcare professionals could hinder market expansion to some extent.
Key Highlights of the Report:
- Peru Urodynamic Equipment Market Outlook
- Market Size of Peru Urodynamic Equipment Market, 2024
- Forecast of Peru Urodynamic Equipment Market, 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Revenues & Volume for the Period 2021 - 2031
- Peru Urodynamic Equipment Market Trend Evolution
- Peru Urodynamic Equipment Market Drivers and Challenges
- Peru Urodynamic Equipment Price Trends
- Peru Urodynamic Equipment Porter's Five Forces
- Peru Urodynamic Equipment Industry Life Cycle
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Product Type for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Urodynamic Equipment for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Urodynamic Consumables for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By End User for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Hospitals for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Ambulatory Centers for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Diagnostic Centers for the Period 2021 - 2031
- Historical Data and Forecast of Peru Urodynamic Equipment Market Revenues & Volume By Others for the Period 2021 - 2031
- Peru Urodynamic Equipment Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By End User
- Peru Urodynamic Equipment Top Companies Market Share
- Peru Urodynamic Equipment Competitive Benchmarking By Technical and Operational Parameters
- Peru Urodynamic Equipment Company Profiles
- Peru Urodynamic Equipment Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















