United States (US) Internal Trauma Fixation Device Market (2025-2031) | Forecast, Trends, Analysis, Segmentation, Companies, Outlook, Competitive Landscape, Growth, Value, Industry, Share, Size & Revenue
| Product Code: ETC9968418 | Publication Date: Sep 2024 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Bhawna Singh | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
United States (US) Internal Trauma Fixation Device Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, the United States continued to see a steady influx of internal trauma fixation device imports, with top exporters including Switzerland, Mexico, Germany, China, and Costa Rica. The Herfindahl-Hirschman Index (HHI) indicated moderate concentration in the market. The compound annual growth rate (CAGR) from 2020 to 2024 stood at a healthy 7.54%, with a notable growth rate of 6.57% from 2023 to 2024. This data suggests a sustained demand for trauma fixation devices in the US market, driven by imports from key exporting countries.
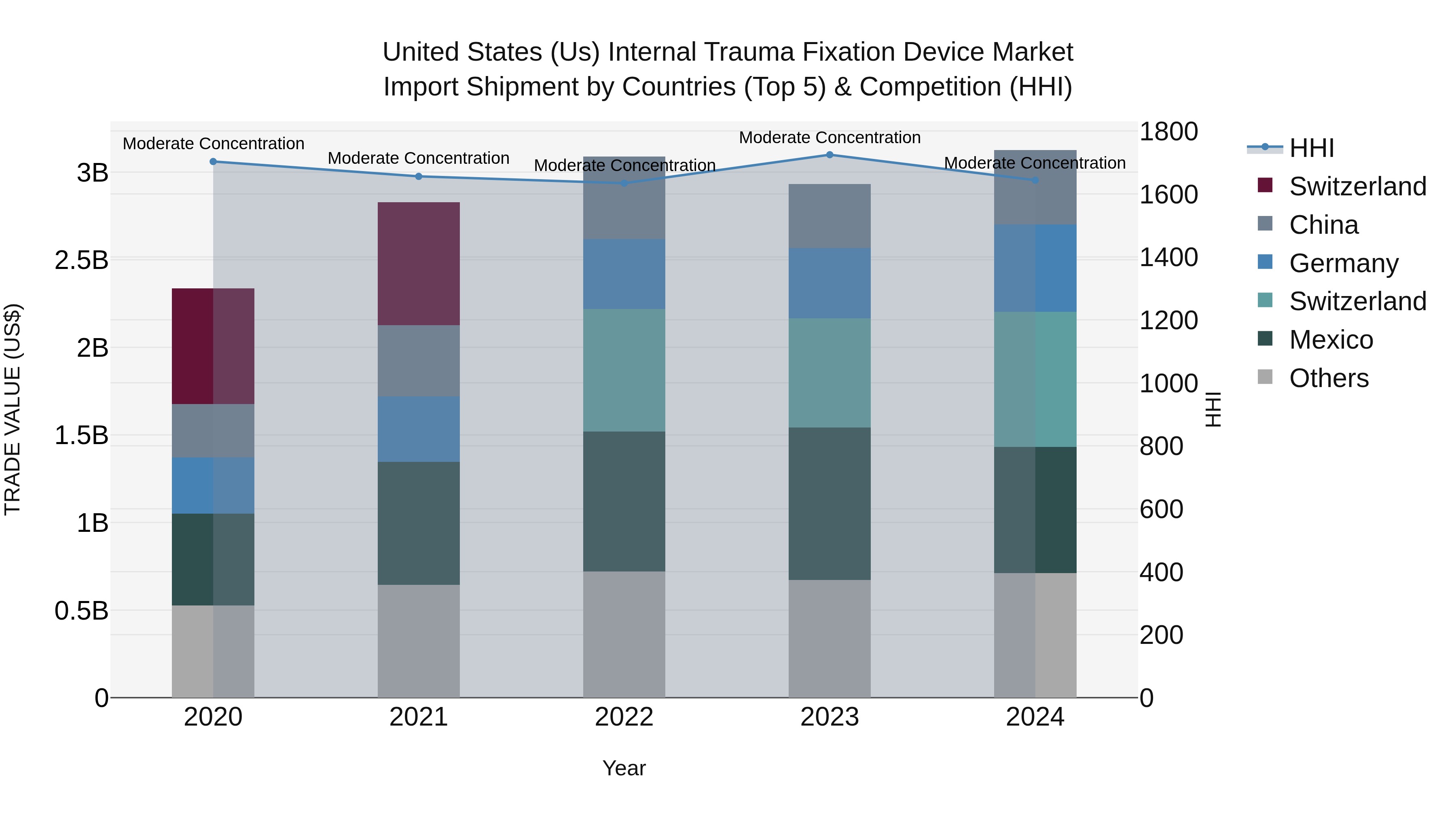
United States (US) Internal Trauma Fixation Device Market Synopsis
The United States Internal Trauma Fixation Device Market is a critical segment within the medical device industry, encompassing a wide range of products used in the surgical treatment of bone fractures and internal injuries. The market consists of various fixation devices such as plates, screws, rods, wires, and nails, which are essential for stabilizing fractured bones and aiding in the healing process. The increasing prevalence of orthopedic injuries due to factors like sports-related activities, road accidents, and an aging population has been driving market growth. Key players in this market include Stryker Corporation, Zimmer Biomet Holdings Inc., and Smith & Nephew plc, among others. Technological advancements, such as the development of biodegradable implants and minimally invasive surgical techniques, are expected to further propel market expansion in the coming years.
United States (US) Internal Trauma Fixation Device Market Trends
The US Internal Trauma Fixation Device Market is experiencing growth due to the increasing incidence of traumatic injuries and road accidents. Key trends include a shift towards minimally invasive procedures, technological advancements in implant materials and designs, and a growing demand for personalized treatment options. Opportunities in the market lie in the development of innovative products such as bioresorbable implants, smart implants with monitoring capabilities, and 3D-printed implants customized for individual patients. The market is also benefiting from the rising geriatric population and the expanding healthcare infrastructure. Companies focusing on research and development to introduce novel solutions that improve patient outcomes and reduce complications stand to gain a competitive edge in this evolving market landscape.
United States (US) Internal Trauma Fixation Device Market Challenges
In the US Internal Trauma Fixation Device Market, challenges include intense competition among key players driving pricing pressures, stringent regulations and quality standards leading to higher compliance costs, and the need for continuous innovation to stay ahead in the market. Additionally, the market faces challenges related to the increasing prevalence of chronic diseases and orthopedic injuries, which drive the demand for trauma fixation devices but also put pressure on healthcare budgets. Moreover, the COVID-19 pandemic has disrupted supply chains and delayed elective surgeries, impacting the overall market growth. To overcome these challenges, companies in the US Internal Trauma Fixation Device Market need to invest in research and development, focus on cost-effective solutions, strengthen distribution networks, and adapt to changing healthcare dynamics to sustain growth and remain competitive.
United States (US) Internal Trauma Fixation Device Market Investment Opportunities
The United States Internal Trauma Fixation Device Market is primarily driven by factors such as the rising incidence of traumatic injuries, increasing sports-related injuries, and the growing elderly population prone to falls and fractures. Technological advancements in internal trauma fixation devices, leading to the development of innovative products with better efficacy and shorter recovery times, also contribute to market growth. Additionally, the expanding healthcare infrastructure and the availability of skilled healthcare professionals in the US further propel market demand for these devices. Moreover, the surge in road accidents and the emphasis on early diagnosis and treatment of fractures and trauma injuries are key drivers shaping the growth trajectory of the internal trauma fixation device market in the United States.
United States (US) Internal Trauma Fixation Device Market Government Polices
The US Internal Trauma Fixation Device Market is significantly influenced by government policies, particularly regulations by the Food and Drug Administration (FDA). The FDA regulates the safety and effectiveness of these medical devices, requiring manufacturers to obtain premarket approval or clearance before selling in the market. Additionally, reimbursement policies set by government healthcare programs such as Medicare and Medicaid impact market dynamics by affecting healthcare providers` financial incentives to use specific devices. The US government also plays a role in funding research and development in the medical device industry, which can drive innovation and market growth. Overall, government policies in the US Internal Trauma Fixation Device Market focus on ensuring patient safety, promoting innovation, and controlling healthcare costs.
United States (US) Internal Trauma Fixation Device Market Future Outlook
The future outlook for the United States Internal Trauma Fixation Device Market is promising, with anticipated growth driven by factors such as an increasing prevalence of traumatic injuries, advancements in technology leading to the development of more innovative fixation devices, and a growing geriatric population prone to fractures. The market is expected to see a rise in demand for internal trauma fixation devices as healthcare facilities focus on improving patient outcomes and reducing recovery times. Additionally, the trend towards minimally invasive procedures and outpatient surgeries is likely to drive the adoption of these devices. Market players are also investing in research and development to introduce new products that offer enhanced performance and better patient outcomes, further contributing to market growth.
Key Highlights of the Report:
- United States (US) Internal Trauma Fixation Device Market Outlook
- Market Size of United States (US) Internal Trauma Fixation Device Market, 2024
- Forecast of United States (US) Internal Trauma Fixation Device Market, 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Revenues & Volume for the Period 2021- 2031
- United States (US) Internal Trauma Fixation Device Market Trend Evolution
- United States (US) Internal Trauma Fixation Device Market Drivers and Challenges
- United States (US) Internal Trauma Fixation Device Price Trends
- United States (US) Internal Trauma Fixation Device Porter's Five Forces
- United States (US) Internal Trauma Fixation Device Industry Life Cycle
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Product for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Closure Device for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Bone Cement for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Material for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Stainless Steel for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Nitinol for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Titanium for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Tritium for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Polyether Ether Ketone (PEEK) for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By End User for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Hospitals for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Orthopedic Surgical Centers for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Trauma Centers for the Period 2021- 2031
- Historical Data and Forecast of United States (US) Internal Trauma Fixation Device Market Revenues & Volume By Ambulatory Centers for the Period 2021- 2031
- United States (US) Internal Trauma Fixation Device Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Material
- Market Opportunity Assessment By End User
- United States (US) Internal Trauma Fixation Device Top Companies Market Share
- United States (US) Internal Trauma Fixation Device Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Internal Trauma Fixation Device Company Profiles
- United States (US) Internal Trauma Fixation Device Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) Internal Trauma Fixation Device Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) Internal Trauma Fixation Device Market - Industry Life Cycle |
3.4 United States (US) Internal Trauma Fixation Device Market - Porter's Five Forces |
3.5 United States (US) Internal Trauma Fixation Device Market Revenues & Volume Share, By Product, 2021 & 2031F |
3.6 United States (US) Internal Trauma Fixation Device Market Revenues & Volume Share, By Material, 2021 & 2031F |
3.7 United States (US) Internal Trauma Fixation Device Market Revenues & Volume Share, By End User, 2021 & 2031F |
4 United States (US) Internal Trauma Fixation Device Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing incidence of road accidents and sports injuries leading to a rise in demand for internal trauma fixation devices. |
4.2.2 Technological advancements in internal trauma fixation devices improving patient outcomes and reducing recovery time. |
4.2.3 Growing awareness among healthcare professionals and patients about the benefits of internal trauma fixation devices. |
4.3 Market Restraints |
4.3.1 Stringent regulatory requirements for product approval leading to longer timelines for market entry. |
4.3.2 High cost associated with internal trauma fixation devices limiting adoption rates. |
5 United States (US) Internal Trauma Fixation Device Market Trends |
6 United States (US) Internal Trauma Fixation Device Market, By Types |
6.1 United States (US) Internal Trauma Fixation Device Market, By Product |
6.1.1 Overview and Analysis |
6.1.2 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Product, 2021- 2031F |
6.1.3 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Closure Device, 2021- 2031F |
6.1.4 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Bone Cement, 2021- 2031F |
6.2 United States (US) Internal Trauma Fixation Device Market, By Material |
6.2.1 Overview and Analysis |
6.2.2 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Stainless Steel, 2021- 2031F |
6.2.3 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Nitinol, 2021- 2031F |
6.2.4 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Titanium, 2021- 2031F |
6.2.5 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Tritium, 2021- 2031F |
6.2.6 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Polyether Ether Ketone (PEEK), 2021- 2031F |
6.3 United States (US) Internal Trauma Fixation Device Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Hospitals, 2021- 2031F |
6.3.3 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Orthopedic Surgical Centers, 2021- 2031F |
6.3.4 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Trauma Centers, 2021- 2031F |
6.3.5 United States (US) Internal Trauma Fixation Device Market Revenues & Volume, By Ambulatory Centers, 2021- 2031F |
7 United States (US) Internal Trauma Fixation Device Market Import-Export Trade Statistics |
7.1 United States (US) Internal Trauma Fixation Device Market Export to Major Countries |
7.2 United States (US) Internal Trauma Fixation Device Market Imports from Major Countries |
8 United States (US) Internal Trauma Fixation Device Market Key Performance Indicators |
8.1 Average length of hospital stay for patients undergoing internal trauma fixation procedures. |
8.2 Number of research and development partnerships established within the internal trauma fixation device market. |
8.3 Adoption rate of minimally invasive techniques in trauma fixation procedures. |
9 United States (US) Internal Trauma Fixation Device Market - Opportunity Assessment |
9.1 United States (US) Internal Trauma Fixation Device Market Opportunity Assessment, By Product, 2021 & 2031F |
9.2 United States (US) Internal Trauma Fixation Device Market Opportunity Assessment, By Material, 2021 & 2031F |
9.3 United States (US) Internal Trauma Fixation Device Market Opportunity Assessment, By End User, 2021 & 2031F |
10 United States (US) Internal Trauma Fixation Device Market - Competitive Landscape |
10.1 United States (US) Internal Trauma Fixation Device Market Revenue Share, By Companies, 2024 |
10.2 United States (US) Internal Trauma Fixation Device Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- South Africa Stationery Market (2025-2031) | Share, Size, Industry, Value, Growth, Revenue, Analysis, Trends, Segmentation & Outlook
- Afghanistan Rocking Chairs And Adirondack Chairs Market (2026-2032) | Size & Revenue, Competitive Landscape, Share, Segmentation, Industry, Value, Outlook, Analysis, Trends, Growth, Forecast, Companies
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















